Species of Aspergillus that produce aflatoxins are ubiquitous and easily isolated from nature. Soil samples are usually diluted through known volumes of sterile water and aliquots plated on solid medium (dilution plated) to obtain quantitative estimates of the population density expressed as colony forming units (cfu)/g in soil. Alternatively, seeds can be plated whole after surface sterilization.
A. flavus and A. parasiticus are not fastidious in their nutritional requirements and will grow on nearly all commonly prepared media for fungi. Isolation of these fungi on agar media instead relies on their sensitivity to certain antibiotics relative to other fungi, their ability to grow at relatively high temperatures (37°C), and their tolerance of low moisture content in the growth medium. The most commonly used media for dilution plating contain the antibiotics Dichloran and or Rose Bengal for restricting fungal colony diameter. The selective medium used to isolate Aspergillus flavus is as under:
Composition of Aspergillus flavus Selective Medium
K2HPO4 - 0.5 g
MgSO4 - 0.5 g
Peptone - 0.5 g
Yeast Extract - 0.5 g
Sucrose - 20.0 g
Agar - 17.0 g
Rose Bengal - 25.0 mg
Streptomycin - 20.0 g
Dist. water - 1000 ml
*Add streptomycin after autoclaving
When culturing for aflatoxin production, single spore isolates of A. flavus and A. parasiticus are necessary to ensure that mixed cultures do not compromise the results. Aflatoxigenic strains may be grown on solid substrates or in liquid media.
Cultivation for identification
Isolates are inoculated at three points on Czapek and malt extract agar (MEA), incubated at 250C. Most species sporulate within seven days. Colour and structure of the conidial head are best observed under a dissecting microscope.
Composition of the media
| Czapek agar | Malt extract agar (MEA) | ||
| NaNO3 | 3.0 g | Malt extract | 20.0 g |
| KCl | 0.5 g | Peptone | 1.0 g |
| MgSO4.7H2O | 0.5 g | Glucose | 20.0 g |
| K2HPO4 | 1.0 g | Agar | 20.0 g |
| FeSO4 | 0.01 g | water | 1000 ml |
| Sucrose | 30.0 g | ||
| Agar | 20.0 g | ||
| water | 1000 ml | ||
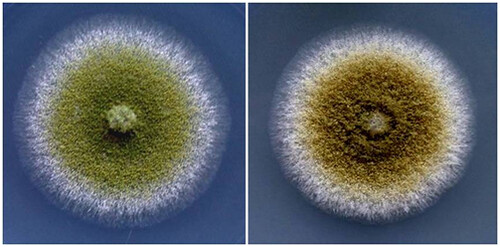
Fig.1: Colony characters of two isolates of Aspergillus flavus from peanut (Gujarat, India)
Identification of aflatoxigenic strains by ammonium hydroxide vapour-induced colour change method
Saito and Machida (1999) introduced a novel method for rapid, sensitive identification of aflatoxin producing and aflatoxin non producing strains ofA. flavus and A. parasiticus. The method was developed empirically and validated using a collection of 120 strains of A. flavus, A. parasiticus, A. oryzae, and A. sojae that were characterized with respect to aflatoxin production by HPLC and UV fluorescence methods. The ammonium hydroxide vapours test is based on the production of yellow anthraquinone biosynthetic intermediates in aflatoxin biosynthesis. These compounds act as pH indicator dyes, which are more visible when they have turned red at alkaline pH.
In this test, fungal colonies are grown on suitable medium such as potato dextrose agar as a single colony in the centre of a Petri dish. The dish is inverted and 1- 2 drops of concentrated ammonium hydroxide solution are placed on the inside of the lid. Very quickly after the bottom of the Petri dish has been inverted over the lid containing the ammonium hydroxide, the undersides of aflatoxin producing colonies turn plum/red. Essentially no color change occurs on the undersides of colonies which are not producing aflatoxins.

A. Low toxic , B. Moderately toxic , C. Highly toxic
Fig.2. Aflatoxigenicity of Aspergillus flavus by ammonia vapour method
Evaluation of peanut samples for Aspergillus flavus seed infection
a) Aspergillus flavus seed infection by blotter plate method
Collect the peanut pod samples and draw the sub-sample lots. Shell the sub-samples and pick randomly 100 undamaged kernels (from each sub-sample) for A. flavus seed infection study by blotter plate method. First soak the kernels in sterile distilled water for 3-5 minutes, then surface sterilize the seed with 1% sodium hypochloride solution for one minute followed by three washes with sterile distilled water. Then place the seed on sterile moist filter paper in the sterile Petri dish. Place all the Petri dishes in moist humid plastic boxes (45 x 25 cm) before they are incubated at 28°C for five days. On sixth day observe the plates and count the number of seed showing A. flavus seed infection.
b) Aspergillus flavus seed infection and colonization by seed plating on solid medium
Surface sterilize the seed as in blotter plate method by sodium hypochlorite followed by three washes with sterile distilled water. Then place the seed gently with the help of forceps onto a solid medium containing potato dextrose agar medium (PDA) in a Petri dish and incubate at 28°C for five days. On sixth day observe the plates and count the number of seed having A. flavus seed infection. Seed colonization may be recorded by cutting the seed by sharp blade/cutter in the middle. Always wear a mask while working with A. flavus seed colonization studies.
In-vitro screening of peanut genotypes for resistance to Aspergillus flavus seed colonization
Select highly virulent, toxigenic strain of A. flavus and multiply it either on PDA or on peanut seed for 7-10 days. Prepare the conidial suspension (1 x 106 spores/ml) to inoculate the seed. Take 100 fully matured undamaged sound kernels from each genotype meant for resistance screening. First soak the seed in sterile distilled water for 3-5 minutes, then surface sterilize the seed with 1% sodium hypochloride for 1 minute followed by three washes with sterile distill water. Then dip the seed in A. flavus spore suspension for 1-2 minute then place the seed on moist blotter in a Petri dish. Place all the Petri dishes in humid plastic box and incubate the plastic boxes at 280C for 5-7 day. After incubation record the number of seed colonized with A. flavus. Precaution should be taken not to damage the seed coat while doing this exercise.
Alternatively, the seed after surface sterilization may be placed on sterile Petri dishes. The conidial suspension (1 x 106 spores/ml) may be put onto the seeds gently a circular motion is given to Petri dish to ensure that enough inoculums get lodged on seed surface. Precaution should be taken not to damage the seed coat while doing this exercise. Then place the seed in proper place maintaining distance between them (10 seeds/plate). Incubate the plastic boxes at 280C for 5-7 day. After incubation record the number of seed colonized with A. flavus.

Fig. 3: In-vitro screening of peanut genotypes (Left- a resistant genotype, and Right- a susceptible genotype)
Screening of peanut genotypes for resistance to Aspergillus flavus infection and aflatoxin contamination under field conditions
Select a highly toxigenic strain of Aspergillus flavus based on their virulence/toxin production. Mass multiply this strain on sorghum or pearl millet grain medium by incubating at 28-300C in the laboratory for 10 days. The field is inoculated thrice by this strain (inoculums of A. flavus multiplied on pearl millet grain medium @ 100 kg/ha) at sowing, flowering and at 90 days of crop. The screening trial is conducted in an isolated plot and inoculation is made each year so as to create a sick plot for Aspergillus flavus and aflatoxin contamination.
Sow the seeds of each genotype to be screened in randomized block design (RBD) or in an augmented plot design in the sick plot. Seed treatment may be given with contact fungicide (Thiram) before sowing. Ensure to impose end season drought/moisture stress towards pod maturity. Dry the pods quickly after harvest.
Observations:
* Soil population of A. flavus
- Before sowing, at flowering (20-25 DAS), 90 days and at harvesting
* Seed infection:
- Take 100 seeds randomly, surface sterilize, put in Petri dishes (5-6 seed/ dish) in moist chamber, incubate at 280C for 4-5 days, take observation of % seed infected.
* Aflatoxin content
- Separate seeds in 4 lots: Bulk, small, medium and large seeded
- Take 20 g seed from each lot and estimate aflatoxin content
References:
Saito M and Machida S (1999). A rapid identification method for aflatoxin producing strains of A. flavus and A. parasiticus by ammonia vapor. Mycoscience 40: 205 -211.
About Author / Additional Info:
Author is a Senior Scientist (Plant Pathology) working at ICAR-National Research Centre on Litchi, Muzaffarpur, Bihar (India). Currently working on diseases of litchi (Litchi chinensis), and mycorrhizal association in litchi. Formerly, he had worked on groundnut (aflatoxins and disease management) at ICAR- Directorate of Groundnut Research, Junagadh, Gujarat (India).