A bio-battery is known as a device in which the substrate material, organic or inorganic, is converted to electric energy. This conversion takes place with the help of various biological or biochemical agents, such as enzymes or micro-organisms.
The substrate is broken down in the presence of these agents to release protons and electrons. The continuous circulation of these protons and electrons within the bio-battery generates electricity.
Need for bio-batteries:
In the field of electricity, a battery is a device that converts chemical energy to electrical energy. Different types of batteries are used in various electronic and electrical devices. However, these batteries contain certain chemicals such as compounds of lead and mercury, which are highly toxic in nature. Also, chemical batteries are prone to explosions, leakages, etc. These problems are not seen in the case of bio-batteries.
Therefore, bio-batteries have a great potential to be used as suitable alternatives or even replacements for chemical batteries in the future.
Types of bio-batteries>
Depending on the type of agents involved in the breakdown of the substrate, the main types of bio-batteries are -
• Enzymatic bio-battery - Biochemical agents, i.e., enzymes are involved in the breakdown of substrate (mainly sugars).
• Microbial bio-battery - Micro-organisms such as Escherichia coli, electric bacteria, etc., are involved in the breakdown of substrate (organic or inorganic).
In both types of bio-batteries, the breakdown of the substrate yields protons and electrons. The circulation of these protons and electrons within the bio-battery generates the conduction of electricity.
Other types of bio-batteries developed include cellulose-based bio-batteries, body fluid-based bio-batteries, etc.
Even mitochondria (sourced from a suitable biological cell) can be used in a bio-battery, since they are regarded as the "energy powerhouses" of the biological cell.
MECHANISM OF WORKING OF A BIO-BATTERY
A bio-battery has three components - anode, separator, and cathode - just like a chemical battery. The separator is filled with an organic fluid which functions as an electrolyte. Two semi-permeable membranes made of cellophane, separate the constituents of the anode, separator, and cathode.
Sources of substrate material (source of energy) -
The sources of the substrate material (source of energy) for the functioning of a bio-battery can be organic (sugars, starch or cellulosic waste, wastewater), or inorganic (metals).
In a bio-battery, sugars are derived from the decay or breakdown of products containing complex carbohydrates such as starch or cellulose (for example, waste paper). Other sources of sugars can be body fluids such as blood, sweat, tears, urine, etc.
• Working of an enzymatic bio-battery -
In an enzymatic bio-battery, sugar-digesting enzymes are immobilized on the anode, and oxygen-reducing enzymes are immobilized on the cathode.
Along with these enzymes, both the anode and the cathode are also immobilized with electron mediators which help in transfer of electrons between enzymes and electrodes. In addition, the cathode is exposed to an environment rich in oxygen, so as to produce water.
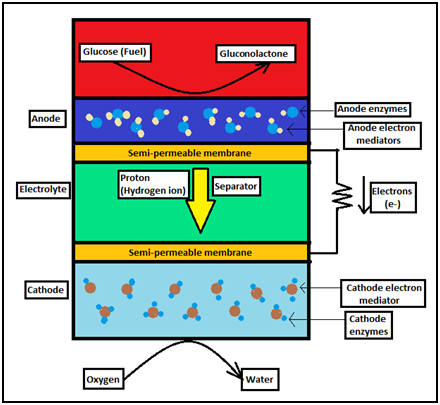
Figure:Enzymatic bio-battery
The concept of the working of an enzymatic bio-battery is similar to the process of respiration in living cells. In living organisms, during the process of respiration, glucose (obtained by consumption of food) is broken down by several enzymes, so as to release energy. Similarly, enzymes break down sugar present in the enzymatic bio-battery, which releases energy. The bio-battery then performs its function on the basis of this released energy.
• Working of a microbial bio-battery -
The microorganisms involved in the functioning of a microbial bio-battery are cyanobacteria (producing carbohydrates through photosynthesis), Escherichia coli, Shewanella oneidensis, etc. These microorganisms are located at the anode, and act as anodic catalysts.
When glucose is broken down at the anode by microorganisms, electrons are released. These electrons are delivered to the cathode. Electricity is generated which can be used to run any electrical device connected to the bio-battery.
- Proteins present on the cell membrane of Shewanella oneidensis have also been known to generate electricity on coming into contact with certain metals such as iron and manganese.
- Electricity has also been obtained from wastewater using microorganisms such as sewage-eating bacteria, which are electrically active. One such example is the microorganism Geobacter sulfurreducens, which also possesses Fe(III)-reducing properties.
- The M13 virus has been genetically engineered such that it produces manganese oxide nanowires for the electrodes of Li-Air batteries used in electric cars. These virus-derived batteries have reported improvement in charging time, and overall functionality.
Reactions involved in a bio-battery -
Consider that glucose is the sugar available for the functioning of the bio-battery. It is broken down for the first time on the anode side of the battery, releasing hydrogen ions (protons) and electrons.
The reaction taking place at the anode is as follows -
The resultant hydrogen ions (protons) are transferred to the cathode via the separator. The resultant electrons are transferred to the cathode through the electron mediator.
At the cathode, a redox reaction takes place. As the cathode is exposed to an oxygen-rich environment, water is produced when oxygen gas reacts with the hydrogen ion (proton) and the electron.
The reaction taking place at the cathode is as follows -
Electric energy is generated by the continuous flow of protons and electrons at the anode and the cathode.
ADVANTAGES OF BIO-BATTERIES
Bio-batteries have various advantages over chemical batteries -
• Quick recharging capabilities - Enzymatic bio-batteries which function on glucose can be recharged quickly due to fast action of the enzymes. Also, in a microbial bio-battery, glucose is an instantaneous source of energy. Therefore, the battery can be recharged extremely quickly. Chemical batteries cannot be charged as quickly as bio-batteries.
• Clean, non-toxic source of energy - Sources of energy (susbstrate material) for the functioning of a bio-battery are completely renewable, non-polluting, as well as environmentally-friendly (wastewater recycled to produce electricity). Therefore, unlike chemical batteries, bio-batteries are a clean, non-toxic source of energy.
• Extremely safe - Bio-batteries do not undergo explosions or leakages, which is not the case with chemical batteries. Therefore, bio-batteries are completely safe to use.
POTENTIAL APPLICATIONS OF BIO-BATTERIES
Presently, bio-batteries are under development for greater improvement, versatility, and usage in various areas. Research is being carried out in this field of study.
However, bio-batteries can have great potential applications in the following fields -
• Electronic devices - Bio-batteries are being developed so as to be used in electronic devices such as laptops and mobile phones. Owing to their quick recharging capabilities, bio-batteries remain ideal replacements for chemical batteries in these devices. Bio-batteries also possess great potential to be used in electronic toys.
• Medicine - Bio-batteries can find great usage in artificially-implanted medical devices such as artificial pacemakers, external hearing devices, battery-operated insulin pumps, etc. Digital thermometers and glucose meters (used by diabetics) can also be operated using bio-batteries.
• Defence purposes - Bio-batteries have great potential to be used in the defence field for the purposes of surveillance, remote sensing, spying devices, etc.
• Fuel synthesis - Scientists have developed a prototype of a solar-powered microbial device (combination of a bio-battery and a solar cell) which produces hydrogen gas. The energy sources for this combination device are wastewater and sunlight. If this technology is further developed and used on a larger scale, wastewater can be efficiently recycled, and the increasing demand for clean, non-polluting fuel can be addressed.
REFERENCES
1. Biobattery http://en.wikipedia.org/wiki/Biobattery
2. Cellulose-based batteries http://advantage-environment.com/framtid/cellulose-based-batteries/
3. Body Fluid Powered Bio-Batteries http://www.lexrobotics.com/body-fluid-powered-bio-batteries/
4. Technology - Bio Battery http://www.sony.net/SonyInfo/technology/technology/theme/bio_01.html
5. Kannan AM, Renugopalakrishnan V, Filipek S, et al - Bio-Batteries and Bio-Fuel Cells: Leveraging on Electronic Charge Transfer Proteins - Journal of Nanoscience and Nanotechnology. 2008, Vol. 8, No. 00 http://www.researchgate.net/publication/24420470_Bio-batteries_and_bio-fuel_cells_leveraging_on_electronic_charge_transfer_proteins/file/32bfe50e458665b76e.pdf
6. Electric Bacteria could be used for Bio-Battery http://www.livescience.com/28163-bio-batteries-one-step-closer.html
7. Electric Bacteria Turn Wastewater Into Energy http://nocamels.com/2011/09/electric-bacteria-turn-wastewater-into-energy/
8. Lithium-air batteries go viral for greater durability and performance http://www.gizmag.com/m13-virus-electrode-lithium-air-battery/29791/
9. Bio-Battery: Clean, Renewable Power Source http://www.cfdrc.com/bio/bio-battery
10. Bio battery based on cellular power plant http://www.rsc.org/chemistryworld/News/2010/August/27081001.asp
11. Could blood be used to power batteries? http://electronics.howstuffworks.com/everyday-tech/blood-battery1.htm
12. Bio Batteries powering implantable bionic devices http://www.electromaterials.edu.au/news/UOW159989.html
13. The Bio-Battery: Converting Sugar into Electrical Energy http://science.dodlive.mil/2010/08/26/the-bio-battery-converting-sugar-into-electrical-energy/
14. Sun and sewage powering up to create hydrogen http://www.ecoseed.org/technology/17165-sun-and-sewage-powering-up-to-create-hydrogen
About Author / Additional Info: