Author: Aditi Mathur
1. What is Enzyme immobilization?
Immobilization refers to restricting the mobility of an enzyme or protein and fixing it into a state without disturbing its functional ability. An immobilized enzyme is fixed to an inert, carrier that not only allows the exchange of substrate and product outflow but also restricts the changes in enzyme conformation due to change in pH or temperature.
Thus enzyme immobilization can reduce the sensitivity of a native enzyme towards these physical parameters hence increasing the functional efficiency of the enzyme. The very first immobilized enzyme was Amino Cyclase from Aspergillus oryzae in Japan.
2. Need of Enzyme Immobilization :
Enzymes are immobilized these days due to several reasons:
- Increased Stability: Since enzymes are fixed to an inert carrier in most stable functional form their resistance to the variation of pH and temperature can be minimized as the sites for sensing these variations are locked in immobilizing the enzyme.
- Economic Nature: Immobilizing enzyme to an inert carrier allows reutilization of enzyme for next batch of reactions. Immobilized enzyme can be easily removed from the reaction mixture thus it reduces the cost of enzyme.
- Easier down streaming: As the catalyst is fixed to a carrier thus down streaming becomes easier as it requires separating only the reactants and final products. Thus it makes the down streaming easier, cheaper and simple.
3. Features of an Ideal Carrier or matrix:
The materials used for immobilization of enzymes are inert matrices and are polymer in nature. they should have following properties :
· Should be cheap
- Inertness
- Stability
- Regenerability after use for next batch
· Tolerant to product inhibition
· Minimum nonspecific adsorption
4. Ways to immobilize an enzyme
There are various ways to immobilize an enzyme.
4.1 Adsorption : It is the simplest and oldest method. It was first used by Nelson and Griffin in 1916 when they immobilized Invertase on an activated charcoal. Enzyme is adsorbed on the physical outer surface of the support. Being a nonchemical method it can affect the functional ability of enzyme by blocking its active / modulating site.
Weak bond or interactions (Hydrogen bond, Vander wall forces) play role in stabilizing the enzyme to carrier.
Carriers used in adsorption can be
- Mineral based support : Aluminum oxide or clay, alginate beads
- Organic Bimolecular based support : Starch , Cellulose
- Modified ion exchange resin : Sepharose
1. Static Method: In this method the carrier is just dipped and left in the enzyme solution without any stirring.
2. Dynamic Method: In this method carrier is placed in the enzyme solution and stirring is maintained to load the enzyme on carrier surface.
3. Reactor loading Method: In this method the carrier is placed in reactor and enzyme solution is loaded with continuous stirring.
4. Electrode position Method: In this method carrier is put in vicinity of an electrode. Now the enzymes migrate to carrier in presence of electric current.
Table 1. Merits & Demerits of Adsorption:
| Merits | Demerits |
| Easy procedure | Weaker attraction thus easily desorbed from carrier |
| No Chemical reagents involved | Less efficient |
| Cheaper method | |
| less disruptive to enzymes |
Table 2.Various chemical groups of Carriers and Enzymes used in bond formation.
| Carrier | Enzyme |
| Amino Group | Alpha Carboxyl at C terminal of Enzyme |
| Hydroxyl Group | Alpha Amino at N terminal of enzyme |
| Carboxyl group | Phenol ring of tyrosine |
| Thiol group | Indole ring of tryptophan |
| Phenol ring | Imidazole ring of Histidine |
- Biomolecules : Carbohydrates are commonly used as a carrier like Cellulose, DEAE Cellulose etc.
- Synthetic Molecules: Synthetic molecules like polyacrylamide are used having good chemical reactivity to form bonds.
- Protein carriers: Protein carriers like Collagen, gelatin are also used.
- Inorganic Molecules: Inorganic carriers include porous glass, silica etc.
1. Diazoation: this reaction occurs between amino group of the carrier and Tyrosil and Histidyl group of the enzyme.
2. Peptide Bond: this is the most common bond formed between amino acids and carrier. It occurs in amino and carboxyl groups of enzyme and carrier.
3. Poly functional Agent: In this method a multifunctional agent like Gluteraldehyde is used to form bond between amino group of enzyme and carrier.
Table 3. Merits & Demerits of Covalent Bonding:
| Merits | Demerits |
| Stronger bond between carrier and enzyme | Chemical changes can destroy native conformation of enzyme |
| No leakage or desorption issue | Inactivation or Reduced efficiency if conformation is changed |
| Wide applicability | |
| Flexible with functional groups |
Matrices used in entrapment method are:
- Polyacrylamide gels
- Agar
- Gelatin
- Alginate
- Carrageen an
1. Inclusion in the gel: In this method enzyme is trapped inside the gel. Gel is formed by the polymer. Pore size of the gel is smaller than that of the enzyme so that there is no leakage. Pore size depends on the concentration of polymer used.
2. Inclusion in fibers: In this method the enzymes are supported on the fibers of the supporting material forming the matrix. The fibers are skeleton of the matrix in which enzyme is trapped.
3. Microcapsules: In this microcapsules are formed in which enzymes are entrapped. Most commonly used polymers in microcapsules are polyamines and sodium alginate.
Table 4. Merits & Demerits of Entrapment:
| Merits | Demerits |
| Faster and cheaper method | Leakage of enzyme |
| Limited or no conformational change of enzyme | Pore diffusion is a limitation both for substrate and product |
| Mild conditions are required | Microbial contamination |
| Easy at small scale application | Limited to small scale operations |
The Physical 3D conformation of enzyme should be retained and sufficient space should be provided for enzyme substrate interaction. To promote the interaction sometimes spacer arms of polyethylene glycol are also used which reduces the stearic hindrances.
Various polymers used for cross linking enzymes are
- Gluteraldehye
- Diazonium salts
| Merits | Demerits |
| Faster and cheaper method | Structural modification |
| Simpler process | Risk of denaturation by poly functional agents |
4.5 Encapsulation: In this method an enzyme is encapsulated within a capsule made up of semi permeable membranes like nitro celluloses, nylon, hemi cellulosic structures etc. In this method the effectiveness depends on the stability of the enzyme inside the capsule...3 D conformation is maintained inside the capsule.
Table 6. Merits & Demerits of Encapsulation:
| Merits | Demerits |
| Faster and cheaper method | Pore size limitation |
| Large amount of enzymes can be encapsulated at a time. | Size of substrate and product a limitation in exchange through membrane. |
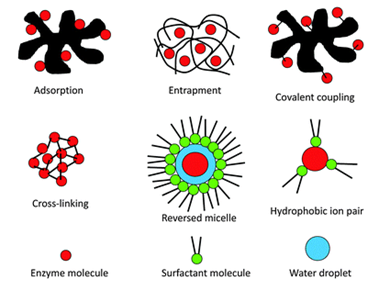
Figure 1: Schematic presentation of enzyme immobilization methods.
4. Advantages & Disadvantages of Enzyme Immobilization
Immobilized enzymes are characterized by various advantages and disadvantages .Some of them is illustrated below.
Table 7. Advantages & Disadvantages of Immobilization methods.
| Advantages | Disadvantages |
| Increases the functional efficiency of enzyme | High cost in isolation and purification of pure enzyme |
| Enzymes are reused | Limited application at large scale |
| Cheaper and economic method | Loss of catalytic function after immobilization |
| Enzymes can be used on continuous mode | Instability increases after immobilization |
| Minimum reaction time | Conformational changes thus reduction in efficiency |
| Less chances of contamination | |
| More stability of products | |
| High enzyme to substrate ratio |
1. Industrial production: Widely used in the commercial production of antibiotics, beverages, amino acids and secondary metabolites etc. of industrial grade.
2. Biomedical applications: Immobilized enzymes are most commonly used in the fast diagnostic kits like ELISA and treatment of many pathogenic diseases.
3. Food industry: Enzymes like Pectinases and Cellulases, amylases are immobilized on suitable carriers or matrices and are successfully used in the commercial production of jams, jellies and syrups from fruits and vegetables. E.g. Lactase is immobilized on cellulose fibers and produce lactose free milk from milk and whey.
4. Large Scale Production of bio-diesel from vegetable oils using bioreactors. They provide a continuous process which reduces the costing to half.
5. Waste water management : In Treatment of sewage and industrial effluents using packed bed reactors .
References:
1. http://www.easybiologyclass.com/enzyme-cell-immobilization-techniques
2. Picture reference : http://pubs.rsc.org/en/content/articlehtml/2013/cs/c3cs35446f
3. Wikipedia.org
4. Biotechnology-Expanding Horizons-B.D Singh
About Author / Additional Info:
I am a post graduate in Botany & Biotechnology.