Authors: Komal Rachandra Pawar1*, Swapnil Gorakh Waghmare2
1VDCOAB, Vasantrao Naik Marathwada Agricultural University, Parbhani-431402
2CPBMB, Kerala Agricultural Univrity, Thrissur-680656
Introduction
In comparison with mammalian cell culture based fermentation technologies plants offer a novel alternative system for the production of pharmaceutical proteins and which is more scalable and cost effective. Plants offer several advantages over current bioreactor based platforms for protein production. Because mammalian cell cultures require a high investment and expensive growth media. One of the most important reason of using plant cells is that they utilize a eukaryotic endomembrane system that is similar to mammalian cells.
There are two methods of gene transfer into plant cell, direct delivery by biolistic and indirect delivery by using Agrobacterium tumefaciens. Direct delivery by biolistic method is also known as microprojectile. In this method gold or tungsten particles are used which is nearly 2 u. By using this method DNA can be introduced in nuclear as well as in chloroplast genome. In indirect delivery by Agrobacterium which naturally transfers the DNA (gene of interest) into plant. T-DNA present in Ti plasmid of Agrobacterium tumefaciens can be replaced by gene of interest which is then transferred into plant cell with the help of vir gene present in Ti plasmid.
Agroinfiltration is a method used to induce transient expression of genes in a plant to produce a desired protein. This method is used in plant biology as well as in plant biotechnology. This method results in 2-5 days. It facilitates rapid evaluation of the expression of genes. Using agroinfiltration multiple genes can be expressed simultaneously. There are two methods of agroinfiltration i.e. direct (syringe) injection and vacuum infiltration. Bacteria uses the mechanism for transfer of DNA to host cell by transferring the T-DNA.
In Agroinfiltration first step is to introduce a gene of interest to a strain of Agrobacterium tumefaciencens. After that strain is grown in a liquid culture to produce large cell mass. This cell mass is washed and suspended into a suitable buffer solution.
First method of agroinfiltration is direct (syringe) infiltration. This method is described by Natorajan et al. (2010) as injecting genetically engineered agrobacteria carrying desired transgene into plants using a needless syringe. In this method for injection this solution is then placed in a syringe and injected into plant leaf. When gentle pressure is applied suspension is delivered into the airspaces inside the leaf through stomata.
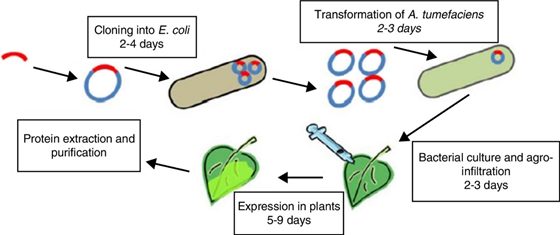
Fig.1 Overview of syringe agroinfiltration
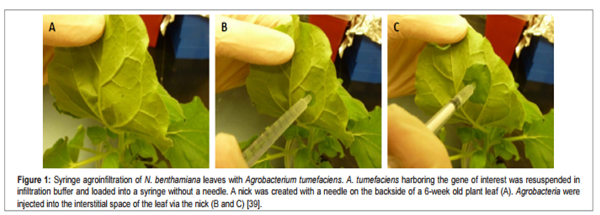
Fig. 2 Syringe agrofiltration
(Chen et al., 2013)
[Image source: http://www.esciencecentral.org/journals/agroinfiltration-as-an-effective-and-scalable-strategy-of-gene-delivery-for-production-of-pharmaceutical-proteins-atbm.1000103.php?aid=14222]
Second method of agroinfiltraton is vacuum infiltration. In this method leaves or whole plants are submerged in a solution and then placed in a vacuum chamber. The vacuum is then applied to make a pressure difference which forces the Agrobacterium suspension into the leaves through stomata into the mesophyll tissue. Agrobacterium remains inside the leaf in the intercellular space and transfers the gene of interest. The gene is then transiently expressed through RNA synthesis from appropriate promoter sequences in all transfected cells. Many plant species can be processed using this method, but most common ones are Nicotiana benthamiana.
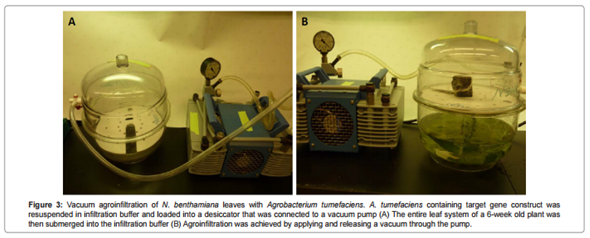
Fig. 3 Vacuum afroinfiltration
(Chen et al., 2013)
[Image source: http://www.esciencecentral.org/journals/agroinfiltration-as-an-effective-and-scalable-strategy-of-gene-delivery-for-production-of-pharmaceutical-proteins atbm.1000103.php?aid=14222]
Infiltration Protocol
(Given by Leuzinger et al., 2013)
A. Syringe Infiltration
1. Take 6-week old N. benthamiana plants with 5 leaves each.
2. Create a small nick with a needle in the epidermis on the back side of the leaf. Attention: make sure not to scratch so hard.
3. Take a firm hold of the front side of the leaf and while applying gentle counter pressure to the nick with the thumb of one hand, inject the Agrobacterium mixtures in infiltration buffer into the nick with a syringe without a needle. Note: As the Agrobacterium mixture enters the intercellular space of the leaf, the infiltrated area will turn visibly darker green.
4. Continue to inject the Agrobacterium mixtures into the nick until the darker green circle stops to expand. Create another nick and repeat above steps until the entire leaf is infiltrated and the whole leaf turns darker green for the first three leaves and the last leaf.
5. After infiltration, move plants back to the growth room and monitor protein expression between 2-15 days post infiltration.
B. Vacuum Infiltration
1. Place a tub into a vacuum desiccator and transfer 3 L infiltration buffer containing the Agrobacterium strains to the tub. Connect the desiccator to a Vacuubrand diaphragm vacuum pump.
2. Place a plant upside down on the desiccator plate and lower the plate with the plant until the entire leaf and stem system is submerged into the infiltration buffer with the plate resting atop of the tub. Place the desiccator O ring along the rim and put the desiccator lid on the chamber.
3. Turn on the vacuum pump and start timing when the vacuum reaches 100 mbar. Slowly open the release valve on the desiccator after 1 min at 100 mbar to allow entrance of Agrobacteria into the interstitial spaces of submerged plant tissue. Repeat this step one additional time to ensure good infiltration.
4. After infiltration, take the plant out of the desiccator and put it back to its upright position. Move plants back to the growth room and monitor protein expression between 2-15 days post infiltration.
The development of this technology will greatly facilitate the realization of plant transient expression systems as a premier platform for commercial production of pharmaceutical proteins.
Qiang et al., (2013) stated that, agroinfiltration based on syringe and vacuum infiltration provides an efficient, robust and scalable gene-delivery technology for the transient expression of recombinant proteins in plants. The development of this technology will greatly facilitate the realization of plant transient expression systems for commercial production of pharmaceutical proteins.
Advantages of (direct) syringe injection
• It is a simple procedure
• It does not require any specialised equipment
• It has the flexibility
Applications
• It is used to Study of plant pathogen interaction
• It has been used for production of abiotic stress tolerance plant
• For the plant gene functional analysis with transient silencing assay
• Protein localization and protein-protein interaction
Advantages of vacuum agroinfiltration
• It has ability to extend gene and protein functional analyses to plant species
• It has enormous scalability potential
• It is more robust
• It can infiltrate a large no. of plants in a short period of time
Applications
• This method is used for large scale production of recombinant proteins by offering advantages in terms of speed, yield and cost.
• Vaccines can be produced against hepatitis B, human papilloma virus, HIVand Influenza virus.
• By using this method antibodies can be produced in the plant.
• In biopharmaceutical industries it has been employed extensively on a commercial scale for recombinant protein production.
• It is ideal option for industrial mass production.
Conclusion
Agroinfiltration is a very good tool to study protein overexpression in the plant. It is an effective method for the gene transfer into the plant. It is a useful tool to boost research in molecular farming.
References:
1. Chen Q, He J, Phoolcharoen W, Mason HS (2011) Geminiviral vectors based on bean yellow dwarf virus for production of vaccine antigens and monoclonal antibodies in plants. Hum.Vaccin 7:331â€"338.
2. Chen Q, Lai H, Hurtado J, Stahnke J, Leuzinger K, et al. 2013. Agroinfiltration as an Effective and Scalable Strategy of Gene Delivery for Production of Pharmaceutical Proteins. Adv. Tech. Biol. Med. 1: 103. doi:10.4172/atbm.1000103
3. Johanna, M. and George, P. L. 2016. Molecular pharming â€" VLPs made in plants. Current Opinion in Biotechnology 37:201â€"206
4. Leuzinger, K., Dent, M., Hurtado, J., Stahnke, J., Lai, H., Zhou, X., Chen, Q. 2013. Efficient Agroinfiltration of Plants for High-level Transient Expression of Recombinant Proteins. J. Vis. Exp. (77), e50521, doi:10.3791/50521
5. Vezina LP, Faye L, Lerouge P, D’Aoust MA, Marquet-Blouin E, Burel C, Lavoie PO, Bardor M, Gomord V. 2009. Transient co-expression for fast and high-yield production of antibodies with human-like N-glycans in plants. Plant Biotechnol J. 7:442â€"455.
About Author / Additional Info:
Research scholar in VDCOAB, Vasantrao Naik Marathwada Agricultural University.