Authors: Rinaldo John1, Varsha Singla2, Meenu Goyal 3, Pardeep Kumar4 and Ankit Singla1,*
1 Department of Industrial Microbiology, Sam Higginbottom University of Agriculture, Technology and Sciences, Allahabad 211-007, Uttar Pradesh, India
2 TFS Lab., Dairy Microbiology Division, National Dairy Research Institute, Karnal 132-001, Haryana, India
3 Department of Biotechnology, Central University of Haryana, Mahendergarh 123-031, India
4 Plant Quarantine Division, ICAR-National Bureau of Plant Genetic Resources, New Delhi 110-012, India
Introduction
Acid drainage problem is associated with the mining of certain minerals that cause long term harm to waterways and biodiversity. Effluents from these mining industries usually contain high quantities of toxic substances such as cyanides and heavy metals which have harmful effects on ecology and the health of living beings. Acid mine drainage (AMD) is acidic water having pH of less than 5, and also containing iron, sulfate and other metals which are formed under natural conditions when hardrock mines containing pyrite are exposed to the atmosphere or oxidizing environments. In other words, AMD can also be stated as outflow of the acidic water from mining sites. Naturally occurring microbes can also increase AMD production by increasing the breakdown of sulfide minerals.
Apart from low pH, the effluents of AMD also have high specific conductivity, high concentration of aluminum and manganese. As the treatment of AMD is expensive and inadequate, it is often left untreated. Pyrite is one of the most important sulfides found in the waste rock of mines. When exposed to water and oxygen, it can react to form sulfuric acid (H2SO4). The following oxidation and reduction reactions express the breakdown of pyrite that leads to AMD. Introduction of hydrogen ions can affect the acidity of a stream. When evaluating the extent of AMD, it is important to know the amount of hydrogen ions remaining in solution after the natural buffering of the stream is completed. AMD depletes the buffering ability of water by neutralizing carbonate and bicarbonate ions to form carbonic acid. Once exposed to AMD, the affected carbonate buffering system is not able to control changes in pH. The buffering system is completely destroyed below a pH of 4.2, where all carbonate and bicarbonate ions are converted to carbonic acid. The carbonic acid readily breaks down into water and carbon dioxide.
Bright orange-colored water and stained rocks are usually tell-tale signs of acid mine drainage. The orange color is caused by ferric hydroxide [Fe(OH)3] precipitating out of the water. The precipitate forms as the AMD becomes neutralized. The metal ions remain soluble at low pH. As the pH rises, the iron oxidizes and precipitates out. Depending on the conditions, the orange-colored precipitates may form inside the mine or several miles downstream.
Sources and factors determining the rate of acid generation
Sources of AMD
There are mainly two sources of AMD. They are: Primary sources and Secondary sources.
- Primary sources : Mine rock dumps Tailings impoundment Underground and open pit mine workings Pumped/ natural discharged underground water Diffused seeps from replaced over burden in rehabilitated areas Construction rock used in roads, dams etc.
- Secondary sources : Treatment of sludge pounds Rock cuts Concentrated load out Stock piles Concentrated spills along roads Emergency ponds
- pH
- Temperature
- Oxygen content of the gas phase, if saturation is less than 100%
- Oxygen concentration in the water phase
- Degree of saturation with water
- Chemical activity of Fe3+
- Surface area of exposed metal sulfide
- Chemical activation energy required to initiate acid generation
- Bacterial activity
Effects of AMD
The major effects of AMD are seen with respect to the aquatic resources. Not much direct impact is seen on humans as long as they are not consuming the water resources affected by deposits of AMD. Many river systems and former mine sites are totally inhospitable to aquatic life with the exception of "extremophile" bacteria. Additionally, heightened acidity reduces the ability of streams to buffer against further chemical changes. The clumps reduce the amount of light that can penetrate the water, affecting photosynthesis and visibility for animal life. Furthermore, when the precipitate settles, it blankets the stream bed, smothering the bottom-dwellers and their food resources.
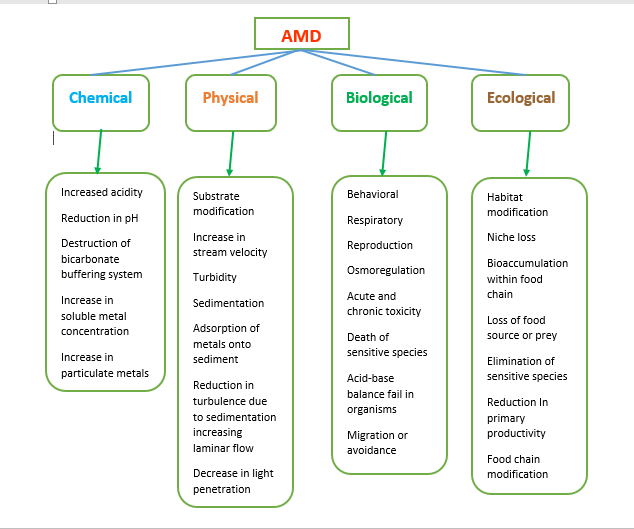
Fig. 1: Negative Impacts of Acid Mine Drainage
Controlling AMD
The major ecological source that could be harmed through AMD is water. Hence, it is important to control the flow of water to mining sites. This could be done by the following measures:
a. Diversion of surface water flowing towards the site of pollution
b. Prevention of groundwater infiltration into the pollution site
c. Prevention of hydrological water seepage into the affected areas
d. Controlled placement of acid-generating waste
The most environmentally effective techniques available for mitigating AMD are internal neutralization methods. The chemicals usually used for AMD treatment include limestone, hydrated lime, soda ash, caustic soda, ammonia, calcium peroxide, kiln dust, and fly ash.
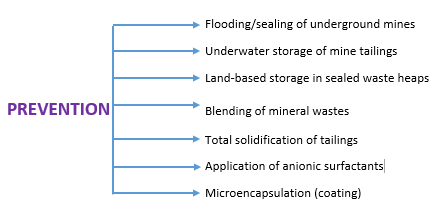
Fig. 2: Strategies for Prevention of Acid Mine Drainage
The alternate ways to mitigate AMD include: prevent sulfuric acid and other acids formation, collect runoff/seepage to contain the acid.
About Author / Additional Info:
Assistant Professor, Department of Industrial Microbiology, Sam Higginbottom University of Agriculture, Technology and Sciences