Authors: Waghmare S. T*., Belge S. A., Yeole P.T., Kharade S. S., Chavan N. S.
Sandeshwaghmare174@gmail.com
Article summary: Abiotic stress is the negative impact of non-living factors on living organism in a specific environment. It is naturally occurring and often intangible factors.Abiotic stresses are the main factors that limit crop productivity. Drought, salinity and heavy metals stresses caused yield losses annually to a greater extent. Transgenic development is another straight forward technology to improve crop yield in abiotic stress affected land. The development of tolerant crops by genetic engineering, on the other hand, requires the identification of key genetic determinants underlying stress tolerance in plants and introducing these genes into crops. The advent of plant transformation may have placed within the grasp of the possibility of engineering greater abiotic stress tolerance in plants.
Introduction:
Plant Genetic Engineering is the transfer of genes from one organisms or even synthetic DNA sequences into the genome of another organism. This capability can be and has been exploited to tailor plant genomics to suit various human needs. One of the best example is the production of transgenic plants which is tolerance to biotic and abiotic stress. A stress is defined as factors of environment interfering the complete expression of the genotypic potential of an organism. It may be biotic stress and abiotic stress. Abiotic stress is the negative impact of non-living factors on living organism in a specific environment. It is naturally occurring and often intangible factors.Abiotic stresses are the main factors that limit crop productivity. Drought, salinity and heavy metals stresses caused yield losses annually to a greater extent. Abiotic stress reducing average yields of crops by up to 50 per cent and annually about 42 per cent of the crop productivity is lost owing to various abiotic stress factors. Transgenic development is another straight forward technology to improve crop yield in abiotic stress affected land. The development of tolerant crops by genetic engineering, on the other hand, requires the identification of key genetic determinants underlying stress tolerance in plants and introducing these genes into crops. The advent of plant transformation may have placed within the grasp of the possibility of engineering greater abiotic stress tolerance in plants.
Genetic engineering for Drought Tolerance
Drought is a period or condition of unusually dry weather within a geographic area where there is a lack of precipitation. Drought is governed by various factors, the most prominent being extremes in temperature, photon irradiance and paucity of water. The characteristics features of drought stress are low water potential due to high solute concentration. Low water supply causes soil mineral toxicities and can make a plant more susceptible to damage from high irradiance.
- Mechanism of drought tolerance
2) DROUGHT AVOIDANCE: It is the ability of plants to maintain relatively high tissue- water potential despite a shortage of soil-moisture. Drought avoidance is performed by maintenance of turgor through roots grow deeper in the soil, stomatal control of transpiration and by reduction of water loss through reduced epidermal i.e. reduced surface by smaller and thicker leaves.
3) DROUGHT TOLERANCE: It is the ability to withstand water-deficit with low tissue water potential. Drought tolerance is the maintenance of turgor through osmotic adjustment (a process which induces solute accumulation in cell), increase in elasticity in the cell and decrease in cell size.
- Barley gene in rice for drought tolerance:
Genetic engineering for Salt / Salinity Tolerance
Salt tolerance is an important trait that requires overcoming salinity induced reduction in plant productivity. The genetic response ofplants to abiotic stresses is complex involving simultaneous expression of a number of genes. Plant genetic engineering techniques could be effectively utilized to exploit some of the untapped potentials to increase the harvestable crop yield. It involves specific genemanipulation either through over expression or silencing of alien/native genes. A number of genes induced in response to salinity have been identified from a range of organisms adapted to stressful environment If a salt tolerant gene is identified which can lead to betterment of the crops, it is possible to transfer that progress in transgenic research for inclusion salinity stress tolerance.
- Yeast gene in Tomato for salinity Tolerance
Genetic Engineering for Heat Tolerance
- Heat-tolerant basmati rice by over-expression of hsp101
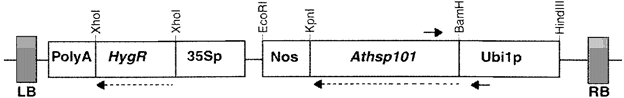
Comparison of survival of transgenic lines after exposure to different levels of high-temperature stress with the untransformed control plants were performed and the result shows that
- The transgenic rice lines showed significantly better growth performance in the recovery phase following the stress
- The results showed that all transgenic rice plants survived in the high-temperature range of 45–50 ◦C exhibiting vigorous growth during the subsequent recovery at 28 ◦C, whereas most of the untransformed plants succumbed.
- These tests revealed that AtHsp101 imparts basal high temperature tolerance possibly by acting in the post-stress recovery period.
Genetic Engineering for Cold Tolerance
Modification of Enzyme
- The chilling sensitivity of plants is closely correlated with the degree of unsaturation of fatty acids.Plants with high proportion of cis unsaturated fatty acids such as Squash and Arabidopsis are resistant to chilling. The chloroplast enzyme glycerol-3-phosphate acetyl transferase seems to be important for determining phosphatidyl glycerol fatty acids unsaturation
References:
1. Roy, B., Noren, S.K.,. Mandal, A. B., and Basu, A.K. (2011). Genetic Engineering for Abiotic Stress Tolerance in Agricultural Crops. Biotechnology, 10: 12
2. Apse, M.P. and Blumwald.E (2002). Engineering salt tolerance in plants. Current Opinion in Biotechnology. 13:146â€"150
3. Bhatnagar, P., Mathur, V. V., and Sharma, K.K.(2008). Transgenic approaches for abiotic stress tolerance in plants: retrospect and prospects. Plant Cell Rep (2008) 27:411â€"424
4. Hussain, S.S., Raza, H., Afzal, M., and Kayani, M.A. (2012) Transgenic plants for abiotic stress tolerance. Current status, Archives of Agronomy and Soil Science, 58:7, 693-721
About Author / Additional Info:
I am currently working as Assistant professor at K.K. Wagh College of Agricultural Biotechnology, Nashik