Authors: Sukumar Taria1, Saroj Kumar Mohanty2 and Manoranjan Kar3
1Ph.D Scholar, Division of Plant Physiology, Indian Agricultural Research Institute, New Delhi- 110012
2Professor and Head, Division of Seed science and Technology, Orissa University of Agriculture and Technology , New Delhi- 110012
3Professor and Vice-Chancellor, Division of Plant Physiology, Orissa University of Agriculture and Technology , New Delhi- 110012
Email-kuna.sj@gmail.com
The chemiosmotic model proposed a key role for a vectorial ATP synthase that would use the energy stored in a proton electrical potential for the synthesis of ATP. This Enzyme was first identified on the basis of its ability to hydrolyse ATP (F-type ATPase).
The thylakoid ATP synthase consist of two segments: a transmembrane hydrophobic segment, called CF0 , and a hydrophilic segment on the stromal surface called, CF1 . CF0 participate in translocating protons across the membrane to catalytic portion of the enzyme, CF1 . CF1 is actually involved in the actual conversion of the ADP and Pi to yield ATP, using the energy stored in the proton gradient. This enzyme is often referred as the CF0 -CF1 complex.
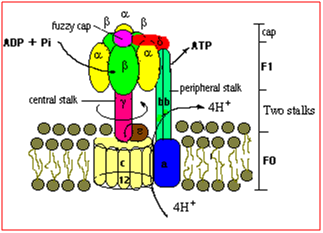
Chloroplast ATP synthase is a 400-KDa enzyme that contains nine different subunits and that includes both chloroplast and nuclear genome encoded gene products.CF1 consists of two relatively large subunits, α and β subunits, each of which has a molecular mass of 50 KDa, plus three smallsubunits called γ , δ and ε. CF1 contain 3 copies of large subunits and one copies of small subunit to give rise to stoichiochemistry of α3β3γδε. The α and β subunits bind ADP and Pi and catalyse the phosphorylation of ADP into ATP. The d subunit links CF0 to CF1, and the ε subunit appear to control the proton gating through the enzyme. The e subunit block the catalysis in the dark, preventing the catalysis of the ATP. The γ subunit also participate in a regulatory mechanism mediated by the ferredoxin/thioredoxin system, which enhances the activation of the ATP synthase in light and deactivation in dark, thereby preventing the wasteful hydrolysis of ATP at night.
CF0 complex consist of four subunits, I, II, III, and IV. These subunits are probably present as single copies, except for subunit III, which is present as 12 copies per complex. These subunits are probably present as single copies, expect for subunit III which is present in about 12 copies per complex. The subunits of the CF0 complex are generally considered to be involved in binding the membrane portion of enzyme to the catalytic portion.
Polypeptide subunit of ATP synthase complex
| PROTEIN | GENE | LOCATION | MOL. MASS | FUNCTION |
| CF1 | ||||
| α- subunit | atpA | C | 55 | catalytic |
| β- subunit | atpB | C | 54 | catalytic |
| γ - subunit | atpC | N | 36 | Proton gating |
| d- subunit | atpD | N | 20 | Binding of CF0 to CF1 |
| ε- subunit | atpE | C | 15 | ATPase inhibition |
| CF0 | ||||
| I | atpF | C | 17 | Binding of CF0 to CF1 |
| II | atpG | N | 16 | Binding of CF0 to CF1 |
| III | atpH | C | 8 | Proton traslocation |
| IV | atpI | C | 27 | Binding of CF0 to CF1 |
C: chloroplast, N: nucleus
In general, 4H+ is consumed per ATP synthesis i.e. 3H+ is translocated through CF0 transmembrane channel and 1H+ is consumed in exchange of Pi through chloroplast membrane.
About Author / Additional Info:
Currently, I am in Ph. D student in Plant Physiology. I am working on abiotic stress management