Authors: Ankit S. Patel1 and Jalpesh S. Patel2
1 Dept. of Agril. Microbiology, B.A. College of Agriculture, Anand Agricultural University
2 Dept. of Biochemistry, B.A. College of Agriculture, Anand Agricultural University
Introduction
Biosurfactants are surface-active amphibolic biomolecules produced by microorganisms. They vary in their molecular size, structure, chemical properties and composition. Biosurfactants possess antifungal, antibacterial and antiviral quantities and also have anti-adhesive action against many pathogenic microorganisms. Types of Biosurfactants: Lipopeptides and lipoprotein, glycolipids, fatty acids, phospholipids, neutral lipids, polymeric biosurfactants, particulate biosurfactants. They are non-toxic or low in toxicity, biodegradable, wastes can be used as raw materials (Walia and Cameotra, 2015).
Lipopeptides
Lipopeptides are types of biosurfactants which have a number of biological activities, including antibiotic, antitumor, immune-modulating and immune-suppressive activities. They are linear or cyclic peptides acylated by a lipid, usually a fatty acid side chain. More than 20 different peptides linked to various fatty acid chains were isolated during 1950s and 1960s from Bacillus sp. Main examples of lipopeptides: Surfactin, Fengycins and Iturins.
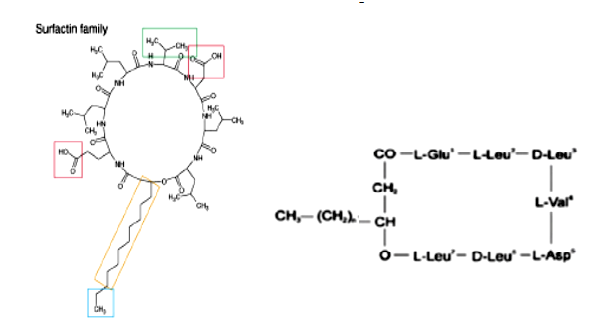
Surfactin
Surfactin is a cyclic lipoheptapeptide. It has the ability to reduce surface tension of water from 72 to 27 mN/m at a concentration as low as 0.005 %. The surfactins are powerful biosurfactants, which show antibacterial activity but no marked fungitoxicity. Surfactin also shows potent antiviral, antimicoplasma, antitumoral anticoagulant activities as well as inhibitors of enzymes.
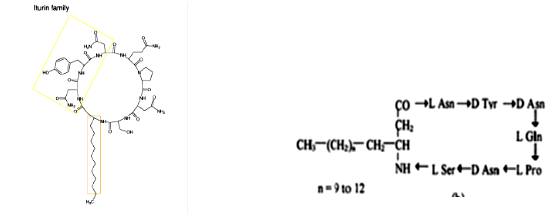
Iturin
Iturin is also a group of cyclic lipopeptides. Iturin A, C, D and E, bacillomycin D, F and L, bacillopeptin and mycosubtilin belong to the iturin group. Special class of pore-forming lipopeptide and are well recognized by their antifungal activity against a wide variety of pathogenic yeasts and fungi. Iturin can increase the membrane cell permeability by the formation of ion-conducting pores. The antifungal activity is related to the interaction of the iturin lipopeptides with the cytoplasmic membrane of target cells and the K+ permeability of which is greatly increased.
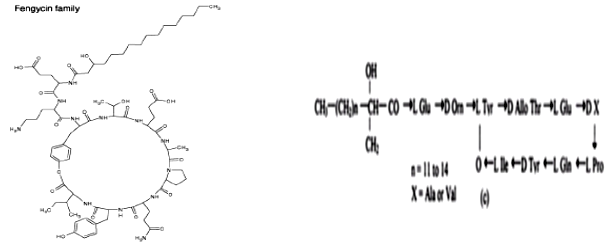
Fengycin
Fengycin are lipopeptides with 10 amino acids and a lipid attached to the N terminal end of the molecule. They are different from iturin and surfactin by the presence of unusual amino acids such as ornithine and allo-threonine. Fengycin family is classified into Fengycin A and B and Plipastatin A and B on the basis of peptide moiety. Like Iturin compounds, Fengycin display strong antifungal activity and inhibit the growth of a wide range of plant pathogens especially filamentous fungi.
Biosynthesis of Lipopeptides
Lipopeptides can be produced by the non-ribosomal biosynthetic pathway, an assembly line that parallels protein synthesis but it is ribosomal-independent and elaborated by a complex mechanism involving multifunctional synthetases. In the biosynthesis of surfactins, combination of enzyme-catalyzed steps are carried out by three modular non-ribosomal peptide synthetases (NRPSs), the surfactin synthetases A, B and C. The enzymatic steps involve aminoacyl-adenylation, thioesterification, condensation, epimerization and thioesterase activities, depending on the specific enzyme module.
Application of Lipopeptides
Ø As Antimicrobial agent (Antibacterial agent, Antifungal agent): Plaza et al., (2013) have studied antifungal and antibacterial properties of surfactin isolated from Bacillus subtilis growing on molasses. They found that surfactin has ability mycelia growth ofBotrytis cinerea A 258 (~50% of inhibition), Sclerotinia sclerotiorum K 2291 (~50% of inhibition), Colletotrichum gloeosporioides A 259 (~40% of inhibition), Phoma complanata A 233 (~38% of inhibition). Das et al., (2007) have observed antimicrobial potential of a lipopeptide biosurfactants derived from a marine Bacillus circulans. They reported that biosurfactant had a potent antimicrobial activity against Gram-positive and Gram-negative pathogenic and semi-pathogenic microbial strains including MDR strains.
Ø As Biocontrol agent: Yanez-Mendizabal et al., (2012) have observed Biological control of peach brown rot ( Monilinia spp.) by B. subtilis CPA-8 is based on production of fengycin-like lipopeptides. They have confirmed by TLC-bioautography that presence of fengycin, iturin and surfactin having strong antifungal activity Monilinia laxa and Monilinia fructicola. Tran et al., (2007) have studied the role of the cyclic lipopeptide massetolide A in biological control of Phytophthora infestans and in colonization of tomato plants by Pseudomonas fluorescens. They found that massetolide A was an important component of the activity of P. fluorescens SS101, since the mass A-mutant was significantly less effective in biocontrol, and purified massetolide A provided significant control of P. infestans, both locally and systemically via induced resistance.
Ø In Biofilm Inhibition: Sriram et al., (2011a) have checked biofilm inhibition and antimicrobial action of lipopeptide biosurfactants produced by heavy metal tolerant strain Bacillus cereus NK1. They found biosurfactant exhibited strong surface activity, significant reduction in biofilm formation by pathogens and showed potent antimicrobial activity against various gram positive, gram negative bacteria and fungi.
Ø In Bioremediation (Hydrocarbon degradation and Heavy metal tolerant): Sriram et al., (2011b) have observed novel lipopeptide biosurfactant produced by hydrocarbon degrading and heavy metal tolerant bacterium Escherichia fergusonii KLU01 as a potential tool for bioremediation. They identified indigenous strain E. fergusonii KLU01 to be a hydrocarbon degrading and heavy metal tolerant bacterium along with the ability to produce lipopeptide biosurfactants.
Ø In Biodegradation of oil: Thavasi et al., (2011) have studied application of biosurfactant produced from peanut oil cake by Lactobacillus delbrueckii in biodegradation of crude oil. They reported that biosurfactants alone is capable of promoting biodegradation to a large extent without added fertilizers.
Ø As Anti-Adhasive potential: Rufino et al., (2011) have observed antimicrobial and anti-adhesive potential of a biosurfactant Rufisan produced by Candida lipolytica UCP 0988. They found that the highest antimicrobial activities were observed against Streptococcus agalactiae, S. mutans, S. mutans NS, S. mutans HG, S. sanguis 12, S. oralis J22 and also showed anti-adhesive activity against most of the microorganism tested.
Ø In Nanotechnology (nanoparticles synthesis): Reddy et al., (2009a) described synthesis of stable gold nanoparticles using sodium borohydrate in the presence of surfactin produced by Bacillus subtilis. They reported the synthesis of stable nanoparticles at pH 7 and 9 remaining intact for 2 months, while aggregation of nanoparticles synthesized at pH 5 occurred within 24 h. Reddy et al., (2009b) used surfactin to stabilize the formation of silver nanoparticles. The nanoparticles were synthesized at different pH conditions and temperature and remained stable for 2 months.
Conclusion
With the changing scenario over the last decade, lipopeptides have proved to be ecofriendly, biodegradable, less toxic and more biocompatible as compared to chemical surfactants. Natural product such as Lipopeptides is used for biological control, antimicrobial and anti-adhesive activities and therefore helps to reduce synthetic chemical compounds. Lipopeptide produced by Bacillus circulans had a potent antimicrobial activity against Gm+ve and Gm-ve pathogenic and semipathogenic microbial strains including MDR strains. B. cereus NK1 was successfully found to be heavy metal resistant and a potent producer of biosurfactants able to emulsify a variety of hydrocarbons and showed substantial antimicrobial activity and biofilm inhibition against various pathogenic infections. Surfactin used for nanoparticle synthesis as a non-toxic and biodegradable stabilizing agent.
References
Das, P.; Mukherjee, S. and Sen, R. (2007). Journal of Applied Microbiology, 104: 1675â€"1684.
Plaza, G. A.; Turek, A.; Krol, E. and Szczyglowska, R. (2013). Afr. J. Microbiol. Res., 7(25): 3165-3170.
Ramarathnam, R.; Bo, S.; Chen, Y.; Fernando, W. G.; Xuewen, G. and Keivit, T. (2007). Can. J. Microbiol. 53: 901-911.
Reddy, A. S.; Chen, C. Y.; Baker, S. C.; Chen C. C.; Jean, J. S.; Fan, C. W.; Chen, H. R. and Wang, J. C. (2009b). Materials Letters, 63: 1227-1230.
Reddy, A. S.; Chen, C. Y.; Chen C. C.; Jean, J. S.; Fan, C. W.; Chen, H. R.; Wang, J. C. and Nimje, V. R. (2009a). Journal of Nanoscience and Nanotechnology, 9: 6693-6699.
Rufino, R. D.; Lunaa, J. M.; Sarubboa, L. A.; Rodriguesb, L. R. M.; Teixeirab, J. A. C. and Campos-Takakia,G. M. (2011). Colloids and Surfaces B: Biointerfaces, 84: 1-5.
Sriram, M. I.; Gayathiri, S.; Gnanaselvi, U.; Jenifer, P. S.; Raj, S. M. and Gurunathan, S. (2011b). Bioresource Technology, 102: 9291-9295.
Sriram, M. I.; Kalishwaralal, K.; Deepak, V.; Gracerosepat, R.; Srisakthi, K. and Gurunathan,S. (2011a).Colloids and Surfaces B: Biointerfaces, 85: 174-181.
Thavasi, R.; Jayalakshmi, S. and Banat, I. M. (2011). Bioresource Technology, 102: 3366-3372.
Tran, H.; Ficke, A.; Asiimwe, T.; Hofte, M. and Raaijmakers, J. M. (2007). New Phytologist, 175: 731-742.
Walia,N. K. and Cameotra, S. S. (2015). J. Microb. Biochem. Technol., 7(2): 103-107.
Yanez-Mendizabal, V.; Zeriouh, H.; Vinas, I.; Torres, R.; Usall, J.; Vicente, A.; Perez-Garcia, A. and Teixido, N. (2012). Eur. J. Plant Pathol., 132: 609â€"619.
About Author / Additional Info:
I am a Ph.D. scholar in Agril. Microbiology with Inspire fellowship