Authors: Pooja Kumari**, S.P. Singh* and K. K. Gangopadhyay*
**Scientist, *Principal Scientist,
ICAR-National Bureau of Plant Genetic Resources, New Delhi-110012
Okra (Abelmoschus esculentus ), belonging to Malvaceae family is originally included in the genus Hibiscus; however, section Abelmoschus is now accepted as distinct genus on the basis of its caducous nature of the calyx (Dhankhar et al., 2005). This genus is important because of two cultivated species, A. esculentus and A. caillei (Patil et al., 2015). Okra is an allopolyploid, having lowest known chromosome number as 2n = 56 in A. angulosus and the highest around 200 in A. caillei, which is an amphipolyploid (allotetraploid) between A. esculentus (2n = 130-140) and A. manihot (2n = 60-68). Among the genus Abelmoschus, A. esculentus is most widely cultivated for its pods throughout Asia and Africa. In the West and Central Africa, A. caillei is cultivated for leaves and pods. A. moschatus is grown as an ornamental plant and also for its aromatic seeds. Okra has been found to possess various ethno-pharmacological and medicinal properties against cancer, high-cholesterol, and Diabetes mellitus. Cultivated okra is mostly susceptible to a large number of begomoviruses having overlapping host range, like radish, tomato, cotton etc. Yellow vein mosaic disease (YVMD), okra leaf curl disease (OLCD), and okra enation leaf curl disease (OELCD) are caused by viruses of genus Begomovirus (family Geminiviridae) resulting in the serious losses in okra cultivation (Venkataravanappa et al ., 2013). Under ï¬eld conditions, infected plants were found to be associated with heavy infestations of the whiteflyBemisia tabaci, the vector of begomoviruses (Venkataravanappaet al., 2015). The loss in yield, due toYellow vein mosaic virus (YVMV) and/or Okra enation leaf curl virus (OELCV) in okra was found ranging from 30 to 100% depending on the age of the plant at the time of infection (Singh, 1996).
Management: the emergence of polyphagous �"B" biotype of Bemisia tabaci, mixed cropping system, along with the increased host range of more than 600 plant species has also resulted in geminiviruses infecting previously unaffected crops. Unlike fungicides and bactericides, no commercial viricides have yet been developed; therefore, viral diseases are not amenable to control by any direct methods. Therefore, management of insect vector is a practical solution. Management of heavy whitefly infestations is quite difficult but prevention is always better than cure. The whiteflies management is possible through utilization of integrated pests management strategies.
- Cultural control: Avoid late sowing and adopt crop rotation with crop which is not the host of whitefly wherever crop rotation is recommended. Proper weeding inside as well as on boundary of the crop is essentially needed. Avoid excess use of nitrogenous fertilizers and do proper drainage of standing water.
- Mechanical control: Hand removal of heavily infested leaves or plants.
- Biological control: Whiteflies have many natural enemies, and outbreaks frequently occur when these natural enemies have been disturbed or destroyed by pesticides, or other factors. General predators include lacewings , bigeyed bugs , and minute pirate bugs . Parasitic wasp, Enacarsia formosa is detrimental to whiteflies. Several small lady beetles including Clitostethus arcuatus (on ash whitefly) and scale predators, such as Scymnus or Chilocorus species, and the Asian multicolored lady beetle, Harmonia axyridis , feed on whiteflies.
- Use of traps: Yellow sticky traps can be used to monitor whiteflies population or, at high levels, reduce whitefly numbers. Traps won't eliminate damaging populations but may reduce them somewhat as a component of an integrated management program.
- Use of botanicals: Neem oil @ 2 ml per litre of water or neem seed kernel extract 5% are useful to eliminate the whiteflies population up to a satisfactory level.
- Use of chemical insecticides: In case of severe infestation spray Triazophos 40 EC @ 1.5 ml per litre of water or Imidacloprid 17.8 SL @ 0.5 ml per litre of water.
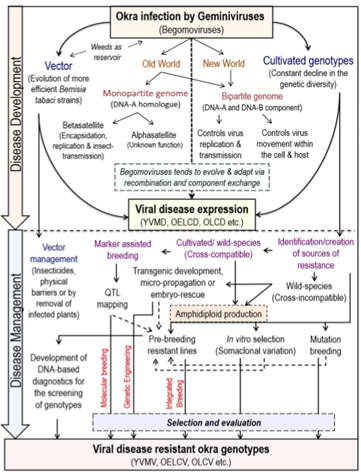
Conclusion: Historically, more than 99% of the worldwide okra cultivation has been localized in the developing countries of Asia and Africa. Until recently, very little attention has been paid to its genetic improvement, and genomic information on Abelmoschus is practically absent.
References:
Dhankhar, S. K., Dhankhar, B. S., and Yadava, R. K. (2005). Inheritance of resistance to yellow vein mosaic virus in an interspeciï¬c cross of okra (Abelmoschus esculentus). Indian J. Agr. Sci. 75, 87-89.
Patil, P., Sutar, S., Joseph, J. K., Malik, S., Rao, S., Yadav, S., et al. (2015). A systematic review of the genus Abelmoschus (Malvaceae). Rheedea 25, 14-30.
Venkataravanappa, V., Reddy, C. N. L., Jalali, S., and Reddy, M. K. (2013). Molecular characterization of a new species of begomovirus associated with yellow vein mosaic of bhendi (okra) in Bhubhaneswar, India. Eur. J. Plant Pathol. 136, 811-822. doi: 10.1007/s10658-013-0209-4
Venkataravanappa, V., Reddy, C. N. L., Jalali, S., Briddon, R. W., and Reddy, M. K. (2015). Molecular identiï¬cation and biological characterization of a begomovirus associated with okra enation leaf curl disease in India.Eur.J.Plant Pathol. 141, 217-235. doi: 10.1007/s10658-014-0463-0
About Author / Additional Info: