Authors: Ankita Hooda, Bimlesh Mann, Rajan Sharma, Sulaxana Singh, Suvartan Ranvir
Ethanol is known for its ability to reduce the colloidal stability of casein micelles and coagulate milk. Indeed, the ethanol stability assays are used as an index of the ability of milk to withstand thermal sterilization. The ethanol stability assay has been used as a tool with which to investigate micelle morphology, and the stability of micelles in ethanol mixtures is important in determining the shelf life of cream liqueurs.
Effect of Ethanol on stearic stability of casein micelles:
Zadow (1993) found out that, at elevated temperatures, ethanol solubilised casein micelles. It was proposed that the reversible dissociation of casein micelles, when these were heated in the presence of alcohol, could be used to incorporate chemicals into the micelles, thereby changing their functional properties. The dissociation of casein micelles when heated to 65 °C in the presence of ethanol [1:1 mixture (v/v) of milk and 65% (w/w) aqueous ethanol was investigated using L* values and transmission measurements. Mixtures of milk and ethanol became transparent on heating, which indicates dissociation of casein micelles. When results of experiments were analyzed by using confocal laser scanning microscopy, light scattering (static and dynamic), to examine the changes of milk during heating in the presence of ethanol, these supported the assertion that such treatments result in dissociation of casein micelles, as did studies of model β-casein micellar systems (Fig. 1).
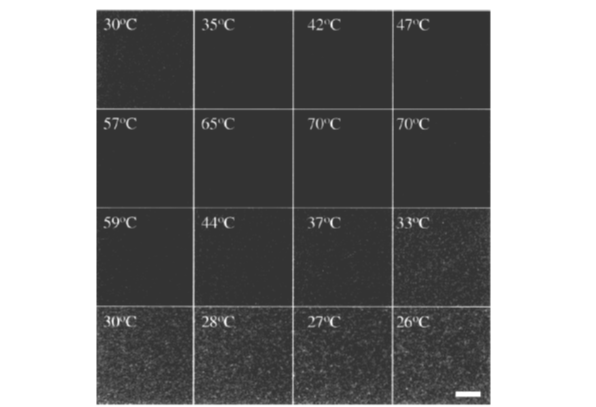
Figure 1: Confocal laser micrographs of a 1:1 (v/v) mixture of milk and 65 % (w/w)aqueous ethanol on heating to 70 C and subsequent cooling. (Connell et al., 2001)
Payens (1979) found out that a component responsible for stearic stabilization was necessary to explain micellar stability. Holt (1975) postulated the existence of a hydrophilic “hairy” coat outside micelle which is responsible for its inherent stability despite low zeta-potential values. Walstra (1979) said that the high voluminosity of the casein micelle could only be explained by assuming the existence of such a hairy surface layer. He also proposed that the hairy part would consist predominantly of the C-terminal portion of K-casein. The action of chymosin would then be to remove the hairs, destabilizing the micelle and simultaneously decreasing its hydrodynamic radius. The latter effect was confirmed by Walstra, who used photon-correlation-spectroscopy (PCS) to show that the micellar radius initially decreased by approximately 5 nm following the addition of chymosin, after which aggregation finally ensued. Coagulation of casein micelles in milk can also be induced by the addition of ethanol. Ethanol stability of milk is based on effects that in an unfavourable solvent environment, proteins and other polymers undergo a conformational transition to a collapsed form, accompanied by a dramatic reduction in the effective volume of the molecule. Solvent conditions which lead to the collapse are often similar to those leading to aggregation. Action of the ethanol in micellar aggregation may therefore be explained as collapse of the “hairs,” & thus removing the stearic stabilizing component from the system.
Horne and co-workers (1981) found out that with no alcohol present, the apparent micelle radius was 93 nm for the milk sample. This decreased to 83 nm in the presence of 16 - 20% ethanol, passed through this minimum, and increased dramatically at higher alcohol levels. The latter increase was time-dependent and was undoubtedly due to aggregation: turbidity also showed a time-dependent increase at these alcohol levels. Below 20% ethanol, as also shown in figure, turbidity values showed a slight increase in the region of minimum radius (Fig. 2). Turbidity is a linear function of the product average molecular weight and average particle-scattering factor. Were the casein micelle to be considered a uniformly dense particle, and were the change in radius due to a shedding of outer layers or to splitting of the micelles, the observed drop in radius would correspond to a change of in molecular weight. Clearly, from turbidity measurements, no such decrease in molecular weight occurred. On the other hand, the slight increase in turbidity at 16 - 20% ethanol suggests an increase in particle-scattering factor which is consistent with a decrease in radius (Fig. 2). The particle-scattering factor is a function of the Z average radius squared.
About Author / Additional Info:
Ph.D scholar in the division of dairy chemistry at NDRI, Karnal