Author: P. Muthukumar
Division of Vegetable Science, IARI, New Delhi-12
Glucosinolates (GLSs) are group of secondary metabolites commonly occurs in Brassica vegetables. Examples of these sources include cabbage, Brussels sprouts, broccoli, cauliflower and various root vegetables (e.g. radish and turnip) the major source of GLSs in the human diet. It contains a variety of glucosinolates, each of which forms a different isothiocyanate when hydrolyzed. They can be grouped into aliphatic, aromatic, and indolic glucosinolates based on their different side chain structures. It is well known that glucosinolates and their degradation products have diverse biological functions that range from anticarcinogenic activities to plant defence against pathogens and herbivores. Prevention and/or protection against chemical carcinogens by phytochemicals present in extensively consumed glucosinolate-containing cruciferous vegetables is of great of interest, as they provide a safe and cost effective means of combating cancer.
Nature and occurrence
GLSs occur in all parts of the plants, but in different profiles and concentrations.
TABLE:1 Trivial and chemical names of Aliphatic GLSs commonly found in the crucifer vegetables
| Trivial names | Chemical names of R-groups | By products | Effects |
| Glucoiberin | 3-methylsulphinylpropyl (3-MSB) | Iberin | Anticancer activity |
| Glucoraphanin | 4-methylsulphinylbutyl (4-MSB) | Sulforaphane | Anticancer activity |
| Sinigrin | 2-Propenyl | - | Anticancer activity |
Metabolism
GLSs are β-thioglycoside N-hydroxysulphates (also known as (Z)-N-hydroximinosulphate esters or S-glucopyr-anosyl thiohydroximates) with a side chain R and a sulphur-linked β-D-glucopyranose moiety (Fig. 1). The sulphate group is normally balanced by a (potassium) cation. Glucosinolates are sulphur rich, nitrogen containing anionic natural products, derived from specific amino acids and their precursors. These glucosinolates are categorized into three classes (aliphatic, indole and aromatic) based on their precursor amino acids and side chain modifications. The side chain R determines whether the GLS is defined as aliphatic, aryl or indole.
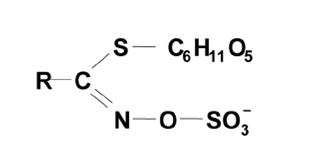
Figure-1 General structure of glucosinolates
Aliphatic glucosinolates are the major group of glucosinolates in Brassica sp, contributing about 90% of the total glucosinolate content of the plant. This glucosinolates derived from methionine, are the most prominent type of glucosinolates in leaves of brassica vegetable. The Myrosinase, enzymes is responsible for hydrolysis of glucosinolates and physically separated from glucosinolates in a intact plant cells. When cruciferous vegetables are chopped or chewed, myrosinase are released to formation of the isothiocyanate (Figure-1). Isothiocyanates are derived from the hydrolysis (breakdown) of glucosinolates-sulphur-containing compounds found in cruciferous vegetables. There are 90 different isothiocyanate (ITC) glucosinolates have been identified only about six occur frequently in the diet. The majority of crucifer vegetables and salad crops have a predominance of alkenyl glucosinolates, the broccoli and calabrese has mainly 3-methylsulphinylpropyl and 4-methylsulphinylbutyl glucosinolates.
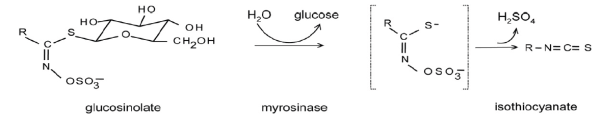
Figure1. Formation of isothiocyanates from glucosinolates
In the family Brassicaceae, the plant's genetic background is the major factor determining GLS concentration and composition, although environmental conditions and physiological factors also influence GLS expression and accumulation. In Brassica vegetables, different species of the same genus and different cultivars of the same species have highly variable GLS concentrations. Table 2 shows the GLS concentration ranges of members of the family Brassicaceae.
Impact on human health
The crucifer vegetables are good source vitamin C, folic acid, vitamin E, carotenoids and flavonoids. It reduces the risk of age-related chronic illness such as cardiovascular health, degenerative diseases and reduces the incidence of certain cancers including bladder, colon, lung and breast in humans. Glucosinolates are converted to isothiocyanates such as sulforaphane and indoles such as indole-3-carbinol (I3C). The aliphatic glucosinolates produces a two bioactive compounds such as 3-methylsulfinylpropyl (Iberin) and 4-methylsulfinylbutyl (Sulforaphane) are derived from the broccoli (Fig. 1), have high anticancer activity (potent inducers of phase II detoxification enzymes in mammalian cell cultures and rodents). The beneficial enzymes such as glutathione-S-transferase and quinone reductase are induced in crucifer vegetables to promote the anticarcinogenic activity. The Indole glucosinolates (those derived from tryptophan) such as Indole-3-carbinol, an hydrolysis product of the glucosinolate glucobrassicin, also proven anti cancer activity.
Major Glucosinolates (GLSs) in commercial crucifer vegetables
| Crucifer vegetables | Glucosinolates groups | Aliphatic glucosinolates (GLS ) Range unit (mg/100 g fw) |
| Green broccoli | Glucoraphanin | 11.6-34 |
| Purple broccoli | Glucoraphanin | 6.7 |
| White cauliflower | Glucoiberin | 0.5-6.6 |
| Green cauliflower | Glucoiberin | 1.2-27.7 |
| Purple cauliflower | Glucoraphanin | 11.6 |
| Glucoiberin | 4.6 | |
| Red cabbage | Glucoraphanin | 4-18.2 |
| Glucoiberin | 4-13.6 | |
| Sinigrin | 3-16.7 | |
| Savoy cabbage | Glucoiberin | 10.4-21.2 |
| Sinigrin | 15.5-18.6 | |
| Brussels sprouts | Sinigrin | 22-25.3 |
| Glucoiberin | 6.4-13.9 | |
| Kale | Sinigrin | 2.2-22.7 |
| Glucoiberin | 13.4-16 |
Conclusion
The great level of variability of GLS content in vegetables existing for the prevention against various type of cancer. Production of high-glucoraphanin enriched lines for particular compounds will boost the drug industry. Improving the value of vegetable as a functional food and increasing the utility of nutritional and medical research. Understanding the bioavailability, transport and metabolism of crucifer vegetables as food is a prerequisite for understanding the mechanisms of their protective effects in humans. The development of broccoli with enhanced concentrations of glucoraphanin may deliver greater health benefits. Research is also needed on the bioavailability and metabolism of GLS to allow recommendations on dietary intake, effective dosage, daily allowance and dietary guidelines for nutrition and health applications.
References
Barbara Ann Halkier and Jonathan Gershenzo 2006. Biology and biochemistry of glucosinolates. Annual Review of Plant Biology Vol. 57: 303-333.
María Elena Cartea and Pablo Velasco. 2008. Glucosinolates in Brassica foods: bioavailability in food and significance for human health. Phytochemistry Reviews. Issue 2, pp 213-229.
Verkerk R, Schreiner M, Krumbein A, Ciska E, Holst B, Rowland I, De Schrijver R, Hansen M, Gerhäuser C, Mithen R, Dekker M. 2009. Glucosinolates in Brassica vegetables: the influence of the food supply chain on intake, bioavailability and human health. Mol Nutr Food Res. Suppl 2:S219.
About Author / Additional Info:
I am currently working as a Scientist (ICAR-ARS) from Indian Agricultural Research Institute.