Authors: Ajay Kumar*, Aman Jaiswal, Deepak Kumar, Shekhar Kumar
Division of Microbiology, ICAR-Indian Agricultural Research Institute, New Delhi (India) 110 012
*Corresponding author e-mail: jangraajay8888@gmail.com
Surfactants
Surfactants, of both biological and chemical origin, are characterized by the formation of aggregates structures such as micelles, their critical micelle concentration (CMC), their potential to decrease interfacial and superficial tensions, their capacity to form emulsions, to solubilize oil, and their foaming and detergent abilities. These properties make them suitable for a variety of industrial, environmental and agricultural applications, such as enhanced oil recovery; wetting agents; antimicrobial, antifungal and antiviral agents; and metal sequestration
Bio-surfactants (BS) are amphiphilic compounds produced on living surfaces, mostly microbial cell surfaces, or excreted extracellular and contain hydrophobic and hydrophilic moieties that reduce surface tension (ST) and interfacial tensions between individual molecules at the surface and interface, respectively.
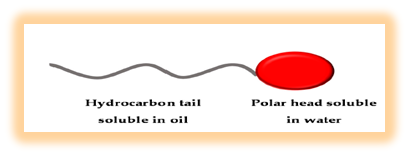
WHY BIOSURFACTANTS…???
- An excessive use of chemical surfactants leads to techno genic load on environment, flora and fauna, and has effects on food products
- Bio-surfactants can satisfy the needs of the modern market in natural products, particularly surface-active substances of new generation (effective and ecologically safe)
| Microbes Type of Surfactant |
| Torulopis bombicola Glycolipid (sophorose lipid) |
| Pseudomonas aeruginosa Glycolipid (Rhamnose lipid) |
| Pseudomonas fluorescens Glycolipid (Rhamnose lipid) |
| Bacillus subtilis Lipoprotein (Surfactin |
| Torulopsis petrophilum Glycolipid & protein |
| Arthrobacter paraffineus Sucrose & fructose |
| Pseudomonas sp. MUB Rhamnose lipid |
RHAMNOLIPID
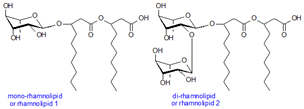
- Rhamnolipids are naturally occuring glycolipids produced commercially by the pseudomonas aeruginosa spp. These are of two types…..
- Mono Rhamnolipid
- Di Rhamnolipid WHY MICROBES PRODUCE RHAMNOLIPID?
- Uptake of hydrophobic substrates
- Antimicrobial properties
- Virulence
- Biofilm mode of growth
- Motility
DETECTION METHODS
1. QUALITATIVE METHOD CTAB agar test
2. QUANTITATIVE METHOD
- Chromatographic method
- Thin layer chromatography
- Gas chromatography
- Liquid chromatography
- Spectrophotometric method
PHYSIOLOGICAL FUNCTIONS AND ROLE OF RHAMNOLIPIDS
Environmental field is considered the most promising market for biosurfactants. Rhamnolipids have been used in various processes such as in bioremediation of hydrocarbons from contaminated soil and water, heavy metal removal, soil washing and oil spills. The process of soil washing is cost effective and relatively fast and has the potential to treat and recover large volumes of contaminants and to prevent the generation of harmful by-products that may be produced during remediation processes. Due to their physicochemical characteristics, rhamnolipids are expected to be more effective than synthetic surfactants and can be blended with other (bio and/or synthetic) surfactants to offer the desired performance characteristics. Some role and function are the following:
- Bioremediation of contaminated agricultural soil
- Application in cosmetic industry
- Plant growth promotion by elimination of phytopathogens and
- Application in pesticide industry
BIOREMEDIATION
“Bioremediation is a waste management technique that involves the use of organisms to remove or neutralize pollutants from a contaminated site.” RHAMNOLIPID HELPS IN BIOREMEDIATION Of
- OIL SPILLS
- INDUSTRY
- AUTOMOBILE
MECHANISM OF RHAMNOLIPID ACTION ON HYDROCARBON
- Emulsifier
- Surfactant
- By attaching the cell wall of bacteria
- Stimulating growth of bacteria(Incr. Biomass)
HEAVY METALS
- Heavy metals & radionuclides are persistent soil contaminants
- They mainly absorb to the surface of soil in the form of ions or the precipitation of metal compound
- Heavy metals are remove from soil through surfactant-associated complexation and ion exchange.
FACTORS AFFECTING RHAMNOLIPID ACTION
- Soil nutrients
- Presense of pesticides
- Soil pH
- Concentration of contaminants
- Soil temperature Rhamnolipids have a big potential, especially in environmental applications for the remediation of contaminated soils due to their biodegradability and low toxicity. Low-cost production and discovery of novel rhamnolipid-producing strains characterized by better yields are the most important keys for rhamnolipids to have a corner on the global market of surfactants.
References
Bordoloi, N.K., Konwar, B.K., 2008. Microbial surfactant-enhanced mineral oil recovery under laboratory conditions. Colloids Surf. B 63, 73–82. Banat, I.M., 1995. Biosurfactants production and possible uses in microbial enhanced oil enhanced oil recovery and oil pollut ion remediation: a review. Bioresour. Technol. 51, 1–12. Ron, E.Z., Rosenberg, E. 2002. Biosurfactants and oil bioremediation. Curr.Opin. Biotechnol. 13, 249–252. Soberón-Chávez G, Lépine F, Déziel E. 2005. Production of rhamnolipids by Pseudomonasaeruginosa. Appl. Microbiol. Biotechnol. 68:718–725. Costa S. G. V. A. O., Nitschke M., Lepine F., Deziel E., Contiero J. (2010). Structure, properties and applications of rhamnolipids produced by Pseudomonas aeruginosa L2-1 from cassava wastewater. Process Biochem. 45, 1511–1516 10.1016/j.procbio.2010.05.033
About Author / Additional Info: