Author: Parimal Ramesh Udgave
Use of Radioisotopes in optimizing fertilizer applications in crops
Fertilizers are primary input for increasing soil fertility and subsequently agricultural production. Fertilizers provide necessary nutrients and minerals to plants which result in proper growth and development of plant. N, P and K are major fertilizers which are Nitrogen, Phosphorus and Potash. Other nutrients such as Boron (B), Sodium( Na), Calcium (Ca), Magnesium (Mg),Manganese (Mn), Chloride (Cl), Iron (Fe),Zn (Zinc),Copper(Cu) and Molybdenum (Mo) also need to be added to maintain and induce proper growth and development.
The table given below includes List of nutrients/Minerals:
Table 1: List of nutrients/Minerals

Excess application of fertilizers often leads to toxic effects to the plants. The effects are given in this article-
Excess application of fertilizers is dangerous for soil health which subsequently leads to poor production of crops. Soil health effects are illustrated below-
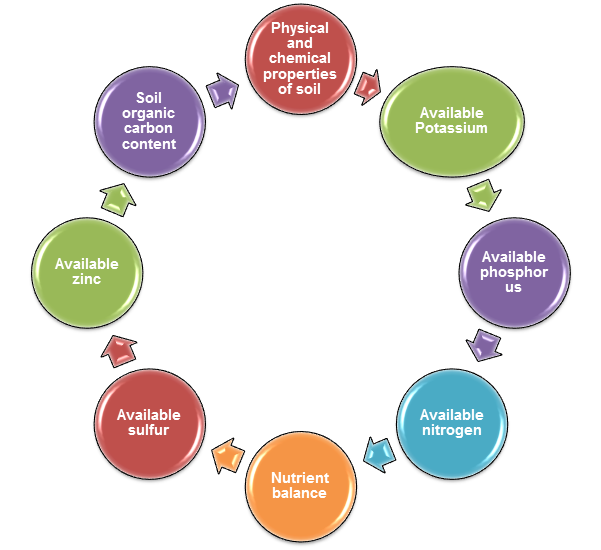
Figure 1: Soil health effects
The above figure shows how fertilizer excess application affects soil which means plant will be affected with the same. Therefore, nutrient-fertilizer balance is very necessary and essential to preserve the quality of soil as well as for more agricultural production.
Second reason behind optimization of fertilizer application is environmental pollution. Higher fertilizer often results in killing soil microbiota which also results in lower agricultural production and affects environmental diversity.
Farmers have big misconception about the fertilizers. One of which is that, the objective of the fertilizer application is to supply nutrients to plant not to the soil. Last concern is economical. Costs of fertilizers can be minimized with their optimal application which can save millions of money for a country.
Optimizing fertilizer or nutrients is now very easy as compared to conventional methods. This method is Radioisotope method.
Isotopic method of calculating fertilizer efficiency
Isotopes are used in this method. Different experiments have been already carried out on nitrogen fertilizers using 15N and Phosphorus fertilizers using 32P and 33P.There is long list to work on different types of fertilizers with suitable isotopes.
Radioisotopes are radioactive isotope of an element which possess unstable atomic nucleus.
An element often has two types of isotope with the different weight. Isotopes can be defined as any of two or more forms of a chemical element, having the same number of protons in the nucleus, or the same atomic number, but having different numbers of neutrons in the nucleus, or different atomic weights.
Example: Phosphorus -32P and 33P
Active radioisotope can be identified using its radioactivity and inactive isotope can be identified by means of mass spectrometry techniques. Therefore, an isotope can be traced out because of its radioactive nature or its mass in physical and chemical changes. However, final choice will be depend on advantages and disadvantages of particular element as tracer.
In the radioisotope method, artificial isotopes are added into fertilizers which can create traceable signals in plant system e.g. in roots, stems, branches, leaves. There are two methods to apply labeled fertilizers which include Pulse (-chase-) labeling and dynamic (Long term) labeling.
In Pulse-labelling method labelled fertilizers are provided for a period of time (pulse), which is very short. Normally grown plants can be utilized for this type of experiments. Normally, in first phase, labeled form of fertilizers becomes incorporated and then it decreases by incorporation of unlabelled form of fertilizers. This can be also called as washout of tracer. Typically, initial observation is made at or shortly after, end of the pulse.
In dynamic method labeling, the labeled fertilizer is supplied continuously during the time course of experiment. The amount of labelled constituent will be rise up to metabolite have reached label saturation. The change in isotopic composition according to time can provide us information how it is converted or carried out in the process.
These are commonly found examples-radioisotopes of nutrient/minerals with half-life
| Radioisotope | Half-Life | ||||
|
| ||||
| Phosphorus-32 | 14.28 hours | ||||
| Copper-64 |
| ||||
| Nitrogen-13 | 10 minutes | ||||
| Zinc-65 | 243.87 days | ||||
| Manganese-54 | 312.1 days |