Authors: N D Saha1*, R Kaur3, Preeti Singh1, Arpan Bhowmik2, Bishal Gurung2 and Eldho Vargese2
1Centre for Environment Science and Climate Resilient Agriculture (CESCRA), IARI, Pusa New Delhi-12
2Indian Agricultural Statistics Research Institute (IASRI), Pusa, New Delhi-12
3 Water Technology Centre, IARI, Pusa, New Delhi-12
*Corresponding Author's email: soilnami@gmail.com
Introduction:
Heavy metals even in trace amount are of major concern because of their toxicity, non-biodegradability and biomagnifying tendency. From different anthropogenic activities, a majority of metals get contaminated in different parts of our environment above the level of permissible limits. Several methods have been devised so far for the treatment and removal of heavy metals from water. The commonly used procedure for removing the heavy metal ions from aqueous streams includes chemical precipitation, lime coagulation, ion exchange, reverse osmosis and solvent extraction. These so-called conventional methods are not so effective when the metal concentration in the effluent is low. These methods are also non-selective.
Adsorption process may be an alternative technology for the removal of heavy metals, when metals are present in very low concentration in the aquatic environment. In the adsorption process, both biosorbents and chemical sorbents can be used for metal removal and recovery. Biosorbents can be prepared involving biological material from forests and plants, ocean and freshwater plankton, algae, fish and all living creatures. Similarly, in chemisorptions process, several adsorbents like zeolite, activated carbon, fly ash, clay and red mud can be used.
Biosorption:
The ability of different living microorganisms to take up heavy metals from aqueous solution was thoroughly studied as early as 18th and 19th centuries but still the applicability of living or non-living microorganisms as adsorbents for removal and recovery of materials from aqueous solutions is proved since last three decades only. The process of biosorption can only be economical if a suitable eluant is used for the recovery of metals from the loaded adsorbent. In a complete metal removal and recovery process, the adsorbents are to be used in a continuous sorption–desorption cycle. For this purpose, the adsorbents should fulfill the following criteria:
- They should be cheap and their easy regeneration is possible
- Both uptake and release of metal ions should be efficient and enough rapid
- Desorption of metal ions from the sorbents should be metal-selective and economically viable. Alginate is such a material which can be used for biosorption purpose. Alginate is a family of linear non-branched polysaccharides and is extracted from brown seaweed. An important part of any biosorption study involves immobilization. Ca-alginate alone has certain capacity to absorb heavy metals. Apart from this, Alginate based beads helps us to immobilize different biosorbent materials very easily. As for example, metal bioremediation capable microbes dead or alive can be easily encapsulated/immobilized into Alginate gels for utilizing in waste water treatment.
Calcium Alginate:
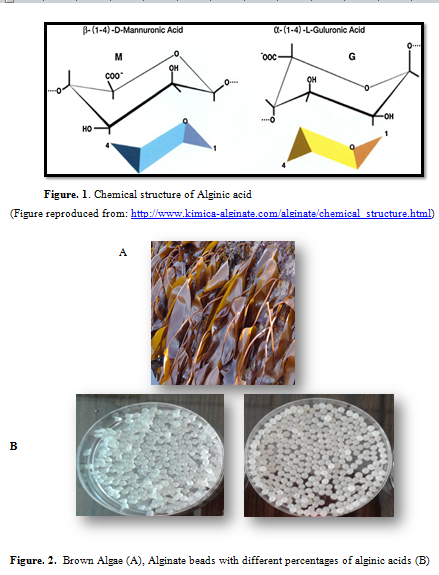
Alginic acid (C6H8O6) n is white to yellow in appearance. It’s a fibrous powder hetero-polysaccharide. Alginic acid is composed of two types of Uronic acid: α-L-Glucuronic acid unit (G) and β-D-Mannuronic acids unit (M) and is found in many algal species especially inside the brown algae (Figure. 1 and Figure. 2 A). Alginate group of chemicals are linear non-branched polysaccharides. It turns into a gel in the presence of divalent and trivalent cations. Alginate is an elastic, irreversible hydrocolloid impression material (Figure. 2 B). Alginates are having multipurpose applications especially in drug delivery systems, cell encapsulation and food industry (Gombotz and Wee 2012). In water treatment, especially to remove heavy metals from waste water, Alginate based biomatrix has immense possibility to play an important role in removing heavy metal ions due to its advantages such as biodegradability, biocompatibility and being environmentally friendly. During adsorption process the heavy metal ions exchange with sodium or calcium ions by ion exchange process (Negm and Ali, 2010). Utilization of alginate sorbents is limited as its mechanical strength and durability are relatively low. But till date many efforts have been given on improvements of mechanical properties of alginates by combining it with other materials like activated carbon, chitosan (Gotoha and Matsushima 2004), polyvinyl alcohol (Han et al. 2012), cellulose and humic acid (Pandey et al. 2003).
Use of Ca-Alginate as bioencapsulation matrix: Ca-Alginate based gel supports many useful bacteria to encapsulate within it. Within the beads the microbes are protected from extreme harsh environment outside yet can perform its remediation and other activities. Different metal resistant beneficial microbial biomass/ live microbes can be easily encapsulated into ca-Alginate based biomatrix and which can be used for waste water treatments (Figure. 3). Each immobilization method has its advantages and disadvantages. The procedure of immobilization in alginate beads is not only inexpensive but also easy to carry out and provides extremely mild conditions, so there is a higher potential for industrial application (Zhou et al. 2010 ). Calcium alginate beads are one of the most commonly used supports for the immobilization of cells. They offer several advantages as a support, such as good biocompatibility, low cost, easy availability, and ease of preparation. However, there are some disadvantages associated with their use, such as gel degradation, severe mass transfer limitations, low mechanical strength (causing cells to be released from the support), and large pore size.

References:
- Gombotz WR, Wee SF (2012) Protein release from alginate matercis. Adv Drug Deliv Rev 64:194–205
- Gotoha T, Matsushima Kikuchia KI (2004) Preparation of alginate chitosan hybrid gel beads and adsorption of divalent metal ions. Chemosphere 55(13):5–140
- Han LCU, Park YU, Kim JA, Kang SB, Lee JK (2012) Immobilization of layered double hydroxide onto polyvinyl alcohol/alginate hydrogel beads for phosphate removal. Environ Eng Res 17:133–138
- Negm NA, Ali HE (2010) Modification of heavy metals uptake efficiency by modified chitosan/anionic surfactant system. Eng Life Sci 10:218–224
- Pandey AK, Pandey SD, Mishra V, Devi S (2003) Role of humic acid entrapped calcium alginate beads in the removal of heavy metals. J Hazard Mater 98:177–181
- Mahmood Z, Amin A, Zafar U, Muhammad A R, Hafeez I, Akram A (2017) Adsorption studies of cadmium ions on alginate–calcium carbonate composite beads. Appl Water Sci.7:915–921
- Zhou Z, Li G, Li Y. Immobilization of Saccharomyces cerevisiae alcohol dehydrogenase on hybrid alginate-chitosan beads. Int J Biol Macromol. (2010) 3:21–26.
About Author / Additional Info: