Heat shock proteins (HSPs) are the most bounteous intracellular proteins which are available in both prokaryotic and eukaryotic cells. On account of their profoundly preserved structure they assumes critical part in major cell forms. HSPs are characterized as atomic chaperone that noncovalently ties with hydrophobic surfaces of nonnative proteins (Csermeley et al., 1998). Its blend is quickly up-managed by different “stressors” including temperature, glucose hardship, contamination and growth. The primary report on HSPs showed up in 1962, after Drosophila salivary organ cells were presented to 37°C for 30 min and afterward came back to their typical temperature of 25°C for recuperation, a puffing of the quality was found to have happened in the chromosome in the recouping cells (Ritossa, 1962). These qualities were therefore found to lead for an expansion in the outflow of proteins of sub-atomic weight of 27-70 kDa. HSPs are limited in the cytosol, mitochondria, endoplasmic reticulum and core inside the cell. Expanded articulation of HSPs has been appeared to be of defensive nature in numerous cells. The insurance is most likely intervened by the HSPs which go about as sub-atomic chaperones and intercedes transport of youthful proteins to the objective organelles for definite bundling, corruption or repairing of the proteins. The part of HSP70 in the collapsing of non-local proteins can be isolated into three related exercises: anticipation of accumulation, advancement of collapsing to the local state and refolding of amassed proteins.
Classification of Heat shock proteins:
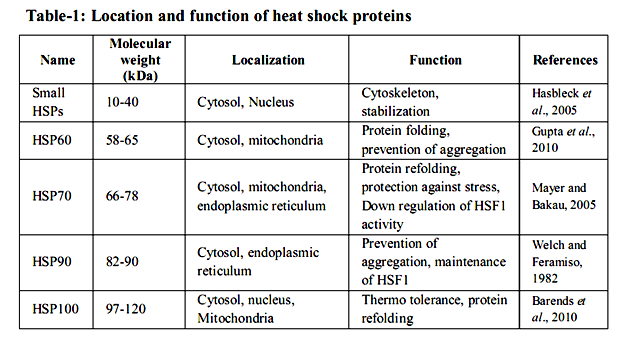
Many studies have tended to the warmth stun reaction on a far reaching level utilizing differential showcase, transcriptional profiling, or proteomic approaches in an assortment of cells and living beings (Eisen et al. 1998; Gasch et al. 2000; Thakurta et al. 2002; Matsuura et al. 2010; Larkindale and Vierling 2008; Richmond et al. 1999; Rohlin et al. 2005; Tabuchi et al. 2008). These studies demonstrated that approximately 50– 200 qualities are altogether actuated in various model life forms, from microbes to human cells. Practically, push inducible proteins can be separated into seven classes. The transcendent class crosswise over species, as far as expression level, are the at first found HSPs, which are no w generally alluded to as sub-atomic chaperones (Ellis et al. 1989). The worthless is spoken to by segments of the proteolytic framework, which are expected to clear misfolded and irreversibly accumulated proteins from the cell. The second rate class is included in curing non physiological covalent changes of nucleic acids. This class incorporates RNA and DNA changing proteins, which are important to repair harmed DNA and handling disappointments that happen amid anxiety (Jantschitsch and Trautinger 2003), for example, the warmth instigated methylation of ribosomal RNAs (Bugl et al. 2000). The fourth HSP class is made out of metabolic chemicals. Here, the variety among species is generally generous. Proof shows that the progressions in pathways amid and after anxiety might be expected to revamp and balance out the vitality supply of the cell (Malmendal et al., 2006; Voit and Radivoyevitch, 2000). The fifth class incorporates administrative proteins like translation components or kinase, some of which are expected to further start stress reaction pathways or to repress expression falls, including ribosome gathering pathways (Refaii and Alix 2009). The 6th class contains proteins required in supporting cell structures, for example, the cytoskeleton. At long last, the seventh class of up-controlled proteins contains transport, detoxifying, and film regulating proteins. These appear to be expected to keep up or reestablish film security and capacity. Heat shock proteins (HSPs) are multi-quality families that extent in sub-atomic size from 10– 150 kDa and are found in all major cell compartments. They are ordered on the premise of their sub-atomic weight and capacity. Five groups of HSPs specifically sHSPs, HSP60, HSP70, HSP90 and HSP100 have singular capacity to perform in different amounts in various cells (table - 1).
HSPs in autoimmune disease:
The possibility that HSPs assume a part in immune system illnesses is not very much bolstered (Kaufmann and Schoel 1994). It has been accounted for that T-cell receptor, stomach muscle positive Tcells can perceive an epitope of HSP65, and this acknowledgment causes immune system sicknesses, for example, adjuvant joint inflammation and non-adjuvant joint pain in mice (Ito et al. 1991, Anderton et al. 1994; Van Eden et al. 1989). Nonetheless, T-cell receptor stomach muscle positive T-cells additionally perceive an alternate epitope of HSP65 and this acknowledgment empowers the tweak of immune system infections in rats (Anderton et al. 1995). The HSP70 may likewise serve as an antigen which is perceived by a subset of T lymphocytes, which communicates g and d chains set up of an and b chains (Tamura et al. 1993; Hisaeda et al. 1997). This T-cell subset is disproportionally expanded in patients with the systemic immune system infection lupus erythematosus. These discoveries proposed that HSP70 assumes a part in immune system ailment (Rajagopalan et al., 1990). Various reports have asserted the nearness of against HSP (e.g., 60 or 70 kDa) antibodies in sera from patients with rheumatic infections, Graves` sickness and Hashimoto`s thyroiditis. Until proof is delivered that unequivocally demonstrates an authoritative part of HSPs in the starting point and pathogenesis of human rheumatic ailments, we ought to consider that the counter HSP antibodies most likely speak to an exertion by the body to shield itself from the HSP-like proteins discharged by focused on cells and irresistible pathogens (Schultz and Arnold 1993).
HSPs in cancer biology:
It has been found that malignancy cells presented at the same time to warm and chemotherapeutic operators bite the dust at a higher rate than cells treated with chemotherapeutic specialists alone. Be that as it may, cells presented to warm before treatment with chemotherapeutic specialists display imperviousness to medication treatment. For instance, refined bosom tumor MCF-7 and MDA-MB-231 cells express large amounts of HSP70 and HSP27 which display resistance for treatment with doxorubicin, actinomycin D, and amphotericin (Ciocca et al. 1982; Shen et al. 1987; Rice and Hahn 1987; Hahn and Li 1990). Obviously the expanded articulation of the multidrug resistance protein intervenes the advancement of imperviousness to numerous malignancy chemotherapeutic operators (Gottesman and Pastan 1988). Strangely, the quality that encodes multidrug resistance protein contains a fitting HSE. Accordingly, regulation of the HSP70 substance may upgrade the restorative adequacy of chemotherapy and maybe permit the utilization of lower measurements of chemotherapeutic operators. Two studies have asserted that specific tumor cell lines express HSP70 on the cell surfaces (Chant et al. 1995; Multhoff et al. 1995). Notwithstanding, these studies have not appeared in an authoritative way that HSP70 proteins are without a doubt layer moored proteins and not simply inexactly connected with the external surface. On the premise of reports that common executioner cells are included in wiping out cell focuses on that express HSP70 (Blachere et al. 1993; Botzler et al. 1996), it is enticing to conjecture that HSP70 immunization may have intense hostile to neoplastic movement (Nakagawa et al. 1991; Rajagopalan et al. 1990; Udono and Srivastava 1993).
HSPs in reproduction biology:
HSP are likewise required in a few procedures fundamental for cell capacity under physiological conditions. HSP generation is upgraded amid in-vitro developing life society and they are among the primary proteins delivered amid mammalian incipient organism development. The unconstrained articulation of HSP as a fundamental piece of incipient organism improvement is all around recorded and nearness or nonappearance of HSP impacts different parts of generation in numerous species. HSPs are immunodominent antigen of various microbial pathogens e.g. chlamudio trachomatis, which have been perceived as the fundamental driver of tubal fruitlessness. The ripeness issues have a past genital tract disease which gets to be sharpened to microbial HSP. A delayed and asymptomatic disease may trigger insusceptibility to microbial HSP epitope that are additionally communicated in man (Neuer, 2000).
References:
1. Anderton SM, Van der Zee, R, Prakken B, Noordzij A, Van Eden W, Activation of T cells recognizing self 60-kD heat shock protein can protect against experimental arthritis, J Exp Med, 181, 943– 952, 1995.
2. Blachere NE, Udono H, Janetzki S, Li Z, Heike M, Srivastava PK, Heat shock protein vaccines against cancer, J Immunother, 14, 352– 356, 1993.
3. Botzler C, Kolb HJ, Issels RD, Multhoff G, Noncytotoxic alkyl-lysophospholipid treatment increases sensitivity of leukemic K562 cells to lysis by natural killer (NK) cells, Int J cancer, 65, 633-638, 1996.
4. Bugl B, Staker BL, Zheng F, Kushner SR, Saper MA, Bardwell JC, Jakob U, RNA methylation under heat shock control, Mol Cell, 6, 349–360, 2000.
5. Chant ID, Rose PE, Morris AG, Analysis of heat-shock protein expression in myeloid leukaemia cells by flow cytometry, Br J Haematol, 90, 163–168, 1995.
6. Ciocca DR, Adams DJ, Bjercke RJ, Edwards DP, McGuire WL, Immunohistochemical detection of an estrogen-regulated protein by monoclonal antibodies, Cancer Res, 42, 4256– 4258, 1982.
7. Csermeley P, Schnaider T, Soti C, Prohaszka Z, Nardai G., The 90-kDa molecular chaperone family: structure, function, and clinical applications a comprehensive review, Pharmacol Ther,79,129– 68,1998.
8. Eisen MB, Spellman PT, Brown PO, Botstein D, Cluster analysis and display of genome-wide expression patterns, Proc Natl Acad Sci USA, 95, 14863– 14868, 1998.
9. Ellis RJ, van der Vies, SM, Hemmingsen SM, The molecular chaperone concept, Biochem Soc Symp, 55, 145–153, 1989.
10. Gasch AP, Spellman PT, Kao CM, Carmel-Harel O, Eisen MB, Storz G, Botstein D, Brown PO, Genomic expression programs in the response of yeast cells to environmental changes, Mol Biol Cell 11, 4241– 4257, 2000.
11. Gottesman MM, Pastan I, The multidrug transporter, a double-edged sword, J Biol Chem, 263, 12163–12166, 1988.
12. Hahn GM, Li GC, Thermotolerance, thermoresistance, and thermosensitization, In: Stress Proteins in Biology and Medicine, Morimoto, R. I., Tissieres, A. and Georgopoulos, C. (eds.) Cold Spring Harbor Laboratory Press, Plainview. 79– 100, 1990.
13. Hisaeda H, Sakai T, Ishikawa H, Maekawa Y, Yasutomo K, Good RA, Himeno K, Heat shock protein 65 induced by gd T cells prevents apoptosis of macrophages and contributes to host defense in mice infected with Toxoplasma gondii, J Immunol, 159, 2375– 2381, 1997.
14. Ito J, Krco CJ, Yu D, Luthra HS, David CS. Preadministration of a 65 kDa heat shock protein, GroEL, inhibits collagen-induced arthritis in mice, J Cell Biochem, 15, 284, 1991.
15. Jantschitsch C, Trautinger F, Heat shock and UV-B-induced DNA damage and mutagenesis in skin, Photochem Photobiol Sci, 2, 899–903, 2003.
16. Kaufmann SHE, Schoel B, Heat shock protein antigens in immunity against infection and Self. In Morimoto R.I., Tissieres A., Georgopoulos, C. Editors, The Biology of Heat Shock Proteins and Molecular Chaperones., Cold Spring Harbour Laboratory Press, Plainview, 495-531, 1994.
17. Larkindale J, Vierling E, Core genome responses involved in acclimation to high temperature, Plant Physiol, 146, 748– 761, 2008.
18. Malmendal A, Overgaard J, Bundy JG, Sørensen JG, Nielsen NC, Loeschcke V, Holmstrup M, Metabolomic profiling of heat stress: hardening and recovery of homeostasis in Drosophila, Am J Physiol Regul Integr Comp Physiol, 291, 205– 212, 2006.
19. Matsuura H, Ishibashi Y, Shinmyo A, Kanaya S, Kato K, Genome-wide analyses of early translational responses to elevated temperature and high salinity in Arabidopsis thaliana, Plant Cell Physiol, 51, 448–462, 2010.
20. Multhoff G, Botzler C, Wiesnet M, Eissner G, Issels R, CD32 large granular lymphocytes recognize a heat-inducible immunogenic determinant associated with the 72-kD heat shock protein on human sarcoma cells, Blood, 86, 1374– 1382, 1995.
21. Nakagawa T, Okano H, Furuichi T, Aruga J, Mikoshiba K, Novel subtypes of the mouse inositol 1,4,5-trisphosphate receptor are expressed in tissue- and developmentallyspecific manner, Proc Natl Acad Sci USA, 88, 6244–6248, 1991.
22. Neuer A, Spandorfer SD, Giraldo P, Dieterle S, Rosenwaks Z, Witkin SS, The role of heat shock proteins in reproduction, Hum Reprod Update, 6, 149-159, 2000.
23. Rajagopalan S, Zordan T, Tsokos GC, Datta SK, Pathogenic anti-DNA autoantibodyinducing T helper cell lines from patients with active lupus nephritis: isolation of CD4282 T helper cell lines that express the T-cell antigen receptor, Proc Natl Acad Sci USA, 87, 7020– 7024, 1990.
24. Rajagopalan S, Zordan T, Tsokos GC, Datta SK, Pathogenic anti-DNA autoantibodyinducing T helper cell lines from patients with active lupus nephritis: isolation of CD4282 T helper cell lines that express the T-cell antigen receptor, Proc Natl Acad Sci USA, 87, 7020– 7024, 1990.
25. Refaii A, Alix JH, Ribosome biogenesis is temperaturedependent and delayed in Escherichia coli lacking the chaperones DnaK or DnaJ, Mol Microbiol, 71, 748– 762, 2009.
26. Rice GC, Hahn GM, Modulation of adriamycin transport by hyperthermia as measured by fluorescence-activated cell sorting, Cancer Chemother Pharmacol, 20, 183– 187, 1987.
27. Richmond CS, Glasner JD, Mau R, Jin H, Blattner FR, Genome-wide expression profiling in Escherichia coli K-12, Nucleic Acids Res, 27, 3821– 3835, 1999.
28. Ritossa F, A new puffing pattern induced by temperature shock and DNP in drosophila, Cellular and Molecular Life Sciences, 18, 571–573, 1962.
29. Rohlin L, Trent JD, Salmon K, Kim U, Gunsalus RP, Liao JC, Heat shock response of Archaeoglobus fulgidus, J. Bacteriol, 187, 6046– 6057, 2005.
30. Schultz DR, Arnold PI, Heat shock (stress) proteins and autoimmunity in rheumatic diseases. Semin, Arthritis Rheum, 22, 357–374, 1993.
31. Shen J, Hughes C, Chao C, Cai J, Bartels C, Gessner T, Subjeck J, Coinduction of glucose-regulated proteins and doxorubicin resistance in Chinese hamster cells, Proc Natl Acad Sci USA, 84, 3278– 3282, 1987.
32. Tabuchi Y, Takasaki I, Wada S, Zhao QL, Hori T, Nomura T, Ohtsuka K, Kondo T, Genes and genetic networks responsive to mild hyperthermia in human lymphoma U937 cells, Int J Hyperthermia, 24, 613–622, 2008.
33. Tamura Y, Tsuboi N, Sato N, Kikuchi K, 70 kDa heat shock cognate protein is a transformation-a ss oc ia t ed a nt i gen a nd a poss ibl e t a r get for t he host ’s a nt i -tumor immunity, J Immunol, 151, 5516–5524, 1993.
34. Terlecky SR, Hsp70s and lysosomal proteolysis, Experientia, 30, 1021-1025, 1994. Thakurta GD, Palomar L, Stormo GD, Tedesco P, Johnson TE, Walker DW, Lithgow G, Kim S, Link CD, Identification of a novel cis-regulatory element involved in the heat shock response in Caenorhabditis elegans using microarray gene expression and computational methods. Genome Res, 12, 701–712, 2002.
35. Udono H, Srivastava PK, Heat shock protein 70 associated peptides elicit specific cancer immunity, J Exp Med, 178, 1391–1396, 1993
36. Van Eden, W, Thole JER, Van der Zee R, Noordzij A, Van Embden JDA, Hensen EJ, Cohen IR, Cloning of the mycobacterial epitope recognized by T lymphocytes in adjuvant arthritis, Nature, 331, 171– 174, 1988.
Voit EO, Radivoyevitch T, Biochemical systems analysis of genome-wide expression data, Bioinformatics, 16, 1023–1037, 2000.
About Author / Additional Info:
I am working as Research Associate in National Institute of Immunology, New Delhi