Authors: Pragyan Paramita Rout
Abstract
Synthetic hydrophilic polymers (hydrogels) are a particular class of gels, obtained by chemical stabilization of hydrophilic polymers in a tridimensional network. Hydrogels are characterized by the ability to absorb and retain quantities of liquids (swelling) much greater, in terms of weight, than the initial weight of the material. Hydrogels have been successfully used as soil improvers to increase the water-holding capacity and/or nutrient retention of sandy soils, with a possible reduction of irrigation frequency, compaction tendency and water run-off. Hydrogels also found applications as slow release fertilizers. Most of the traditional hydrogels on the market are acrylate-based products, thus not biodegradable and regarded as potential pollutants for the soil. Due to the increasing attention for environmental protection issues, biodegradable hydrogels arise lively interest for potential commercial application in agriculture.
Introduction
In current Indian agricultural scenario, about 70 percent of 143 million hectare of total cultivated area is under rain fed agriculture. The rain fed areas contributes for 42 percent of the total food grain production wherein, water management is a critical factor that limits crop production, due to uncertainty in climate. To address such moisture scarcity, superabsorbent nano-hydrogel seems to be a novel agri-input in the field of nano-technology which has the capacity to absorb large volume of water within short period of time and desorb the absorbed water under stress condition.
Nano-hydrogels and its types
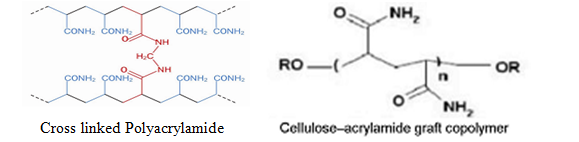
Superabsorbent nano-hydrogels are a particular class of gels, obtained by chemical stabilization of hydrophilic polymers in a tridimensional network. Hydrogels can be identified as hydrophilic polymeric materials with network structures and appropriate degree of crosslinking. These are classed into three categories based on the nature of monomers (Woodhouse and Johnson, 1991)
- Starch-polyacrylonitrile graft polymers (starch co-polymers)
- Vinyl alcohol-acrylic acid co-polymers (polyvinyl alcohols)
- Acrylamide sodium acrylate co-polymers (cross-linked polyacrylamides)
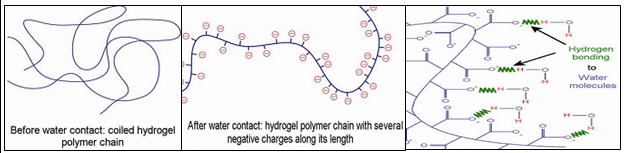
Water absorption mechanism of hydrogels
The swelling behavior and water absorption capacity of hydrogels are the most important properties that give wide applications in rain fed agriculture to mitigate water stress. Swelling and absorption properties are mainly attributed to the presence of hydrophilic groups such as acrylamide, acrylic acid, acrylate and carboxylic acid present in the polymeric network. When these polymers are put in water, the acid functional group enters into the hydrogel structural backbone by osmosis, hydrogen atoms react and come out as positive ions. This leaves negative ions along length of the polymer chain. These negative charges repel each other and forces polymer chain to unwind and open up. These in turn, attract water molecules and bind them with hydrogen bonding (Fig 2).
Hydrogels are called as super absorbents which hold 400-1500 g of water per dry gram of hydrogel (Liang et al., 2009). When these absorbents are mixed with the soil, an amorphous gelatinous mass is formed on hydration. They are capable of cyclical absorption and desorption for long period of time. This cyclic process can last up to 2-5 years in soil followed by decomposition of hydrogels.
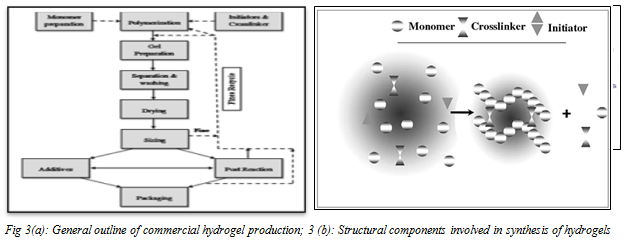
Technologies adopted in hydrogel preparation
In general, hydrogels can be prepared from either hydrophilic synthetic or natural polymers. The synthetic polymers are hydrophobic in nature and chemically stronger compared to natural polymers. Copolymerization/cross-linking free-radical polymerizations are commonly used to produce hydrogels by reacting hydrophilic monomers with multifunctional cross-linkers. Water-soluble linear polymers of both natural and synthetic origin are cross-linked to form hydrogels via several polymerization reactions such as
- Solution polymerization
- Free radical polymerization
- Photo polymerization
- Chain transfer polymerization
- Suspension polymerization
- Radiation polymerization
Functional features of ideal nano-hydrogels
- High absorption capacity (maximum equilibrium swelling)
- Low soluble content and residual monomer
- High durability and stability in swelling environment and during storage
- Biodegradability without formation of toxic species
- pH-neutrality after swelling in water
- Non-toxic and photo stable
Hydrogel has been used to improve water retention of soil and water managing materials for degraded land. These have been widely proposed over the last 40 years for agricultural use with the aim to ameliorate water availability for plants, by increasing water holding properties of growing media (soil or soilless substrates). Most of the traditional hydrogels on the market are acrylate-based products, thus not biodegradable and regarded as potential pollutants for the soil. Due to the increasing attention for environmental protection issues, biodegradable hydrogels arise lively interest for potential commercial application in agriculture (Liang et al., 2009). Hydrogels also found applications as slow release fertilizers. Slow release fertilizer hydrogel (SRFH) is a combination of hydrogel and fertilizer mainly created to aid slow release of nutrients, reduce evaporation losses and frequency of water irrigations.
Agricultural hydrogel products available in India

Application methods and rates
For field crops: Prepare an admixture of hydrogel and fine dry soil in 1 : 10 ratio and apply along with seeds/fertilizers or in the opened furrows before sowing.
In nursery bed for transplants: Apply 2 g/m2 (or according to recommended rate) of nursery bed mix of hydrogel uniformly in the top 2 inches of the nursery bed.
Transplanting: Thoroughly mix 2 g (or according to recommended rate) of hydrogel per liter of water to prepare a free-flowing solution; allow it to settle for half an hour. Dip the roots of the plant in the solution and then transplant in the field.
In pot culture: mix 3-5 g/kg of soil before planting.
Fate of hydrogels in environment
Biodegradable hydrogels contain labile bonds either in the polymer backbone or in the cross-links used to prepare the hydrogels. The labile bonds can be broken under physiological conditions either enzymatically or chemically over a period of time. End-products after degradation are CO2, water and ammonia. Acrylamide, a monomer used for hydrogel preparation is neurotoxic, but polyacrylamide itself is non-toxic. The polyacrylamide can never reform its monomer. Hence there is no residual amount of acrylamide present in the soil after degradation of hydrogel, especially when cellulose is used as backbone. Acrylamide residue is also not detected in crop products which are grown with hydrogel application.
Conclusion
Hence, nano hydrogels created an attractive area in the viewpoint of super-swelling behavior chemistry under drought stress in rain fed areas and its production via replacing synthetics with bio-based materials e.g., polysaccharides and polypeptides at commercial scale need attention to achieve environment stewardship.
References
1. Woodhouse, J.M. and M.S. Johnson. 1991. The effect of gel-forming polymers on seed germination and establishment. Journal of Arid Environment., 20: 375-380.
2. R. Liang, H. Yuan, G. Xi and Q. Zhou. 2009. Synthesis of Wheat Straw-G-Poly (Acrylic Acid) Superabsorbent Composites and Release of Urea from It. Carb Pol., 77: 181-187.
About Author / Additional Info:
I am pursuing PhD in Soil Science from Tamil Nadu Agricultural University, Coimbatore. Currently I am doing my doctoral research work on "standardization of moisture sensors and precision nutrient management in flower crops"