Authors: Jalpesh S. Patel*, Vyomesh Patel and J. J. Dhruve
Department of Biochemistry, B.A. College of Agriculture, Anand Agricultural University, Anand, Gujarat, India-388110
*Email: jalpeshpatel.2729@yahoo.com
Abiotic stress and its types
Stress is external condition that adversely affect on plant growth, development and productivity. The negative impact of living factors and non-living factors on the living organisms in a specific environment is known as Biotic stress and Abiotic stress respectively. The major factors of abiotic stress are high temperature, low temperature, water stress, water lodging, various salt, oxidative, deficiency of nutrients, excess of heavy metals etc.
Cellular response during abiotic stress
Increase reactive oxygen species (ROS) scavenging activities in cytoplasm and organelles. Accumulation of sugars, sugar alcohols and free amino acids in cytoplasm. It leads to increase of classes of proteins having repair functions, like heat shock proteins (HSPs)/chaperones, dehydrins, late embryogenesis abundant (LEA) proteins. Increase concentration of osmolytes viz., total soluble sugars, free amino acids, sorbitol, phenols, proline etc. as well as polyamines (PAs) accumulated (Bohnert 2007).
Polyamines in plants
Polyamines are nitrogen containing organic molecules, discovered in human semen by Antoni van Leewenhoek in 1678. They are strongly basic in nature having straight-chained aliphatic hydrocarbons C3â€"C15 (Luo et al., 2009). Polyamines are distributed in all vegetative and reproductive plant organs like roots, stems, leaves, flowers, seeds, xylem, and phloem. They are localized mainly in vacuoles, also bound to organelles like nuclei, mitochondria, chloroplasts, ribosome, cell-walls, and membranes (Edreva, 1996).
Plant polyamines are classified in to free polyamines and conjugated polyamines. The free polyamines in plants are Di-amine (Putrescine, cadaverine, 1,3-diaminopropane), Tri-amine (homospermidine, Spermidine, norspermidine), Tetra-amine (Spermine, norspermine, canavalmine) Penta-amines (aminopropylcanavalmine, aminobutylcanavalmine, caldopentamine) (Edreva, 1996). The conjugated polyamines in plants are caffeoylputrescine, feruloylputrescine, coumaroylagamatine, feruloylagamatine, feruloyltyramine, coumaroylputrescine (Walters, 2003).
Anabolism of common Polyamines
The biosynthesis of common Pas can be divided in to two main steps:
• Synthesis of the diamine putrescine
• Formation of spermidine (Spd) and spermine (Spm) fromputrescine
Putrescine is synthesized by two main pathways in plants. It can be formed directly by decarboxylation of L-ornithine, in a reaction catalyzed by ornithine decarboxylase (ODC) and decarboxylation of L-arginine by arginine decarboxylase (ADC). Spd and Spm synthesis is carried out by addition of an aminopropyl group to one or both primary amino groups of Put by Spd and Spm synthases respectively. Decarboxylated S-adenosylmethionine (DSAM) is the donor of aminopropyl group which is derived from S-adenosylmethionine (SAM) in a reaction catalyzed by SAM decarboxylase (Palavan, 1995).
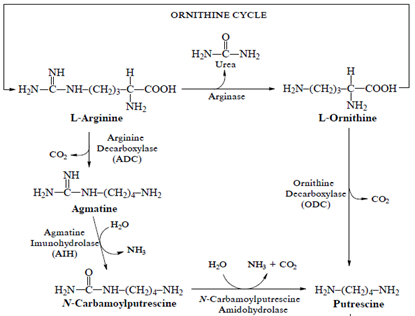
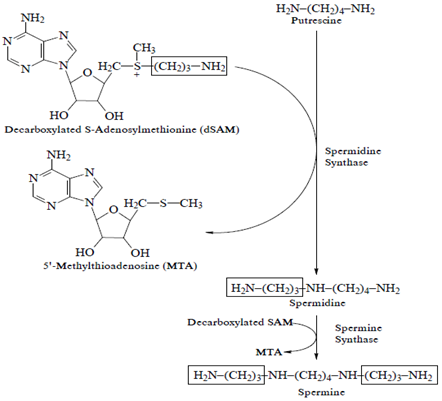
Fig.1: Biosynthesis of putrescine, spermidine and spermine
Catabolism of common Polyamines
Putrescine is degraded by the diamine oxidase (DAO) and form 4-aminobutanal, Spd is degraded by polyamine oxidase (PAO) and form 4-aminobutanal and 1,3diaminopropane, while Spm is degraded by PAO and form N-(3 aminopropyl) Pyrrolineand 1,3 diaminopropane (Liu et al., 2007).
Interactions of Polyamines with other metabolic routes Alcazar et al. (2010) suggested the following link between polyamines and other metabolic route
• PAs and ethylene are connected through common precursor SAM
• H2O2 generated by the action of DAO and/or PAO is involved induction of Abscisic acid
• Proline and polyamine share ornithine as a common precursor
Polyamines and abiotic stress
Plant polyamines are preferentially detected in actively growing tissues and under stress conditions. PAs involvement in abiotic stresses adaptation is due to their roles in osmotic adjustment, inhibit lipid peroxidation, cell-wall strengthening, membrane stability, scavenging free radicals, induces antioxidative enzymes, affecting nucleic acids and protein synthesis, interacting with hormones and ethylene biosynthesis (Pang et al., 2007, Araby et al., 2011).
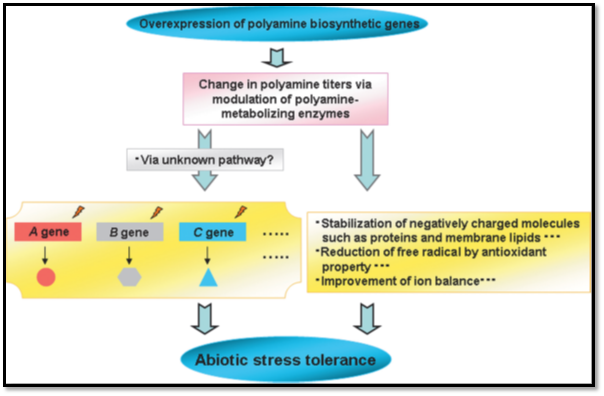
PAs include electrostatic binding to macromolecules. PAs interact with ion channels and receptors, resulting in regulation of Na+, K+ and Ca+2 homeostasis. Production of H2O2 (Hydrogen peroxide) upon polyamine catabolism helps in the induction of abscisic acid. Putrescine is an efficient stimulator of ATP synthesis and causes depolarization of membranes. Spermidine and spermine also prevent leakage of amino acids and electrolytes. PAs are reversing stress induced damages in photosynthetic apparatus and help in photosynthesis.
Legocka and Kluk (2005) have reported the effect of NaCl (260mM) and sorbitol (360mM) on changes in polyamine content and ADC activity in Lupinus luteus seedlings. After exposing the plants to NaCl and sorbitol for 4 h, concentration of total polyamines increased. But when plants were exposed for 24 h, the level of free Put decreased in roots and cotyledons, and increased in hypocotyls and leaves. The level of free Spd also decreased in roots, in contrast to the increase of Spd observed in hypocotyls and leaves. In the presence of NaCl and/or sorbitol, the activity of ADC in roots increased by about 66% and 80%, respectively that indicates the translocation of polyamines from roots to leaves. Yamaguchi et al. (2006) studied that the polyamine Spm protects against high salt stress in Arabidopsis thaliana wild type and double knockout mutant plant (acl5/spms). The mutant showed higher sensitivity to high salt than wild type plants. This phenotype was cured by exogenous spermine but not by any other polyamines putrescine and spermidine, which suggesting a strong link between spermine deficiency and NaCl hypersensitivity.
Cheng et al., (2009) observed the polyamine accumulation in transgenic tomato enhances the tolerance to high temperature stress. SAMDC cDNA isolated from Saccharomyces cerevisiae was introduced into tomato genome by means of Agrobacterium tumefaciens through leaf disc transformation. Transgenic plants expressing yeast SAMDC produced 1.7 to 2.4 fold higher levels of Spd and Spm than wild-type plant under high temperature stress, and enhanced antioxidant enzyme activity. Kasukabe et al., (2004) examined that overexpression of spermidine synthase enhances tolerance to multiple environmental stresses in Arabidopsis thaliana. The cloned spermidine synthase cDNA from Cucurbita ficifolia gene was introduced to Arabidopsis thaliana under the control of the cauliflowermosaic virus 35S promoter. As compared with the wild-type plant, the T2 and T3 transgenic plants exhibited a significant increase in spermidine synthase activity and Spd content in leaves together with enhanced tolerance to various stresses including chilling, freezing, salinity, hyperosmosis, drought.
Conclusion
Abiotic stress condition increases the levels of free and conjugated polyamines. Polyamineshelp in scavenging the free radicals and induce activity of antioxidant enzymes. Polyamines help in osmotic adjustment and also in maintaining ionic balance. Salt stress increase the activity of DAO in salt susceptible plant .Induction of abscisic acid during various abiotic stress cause the over expression of polyamine synthesis genes like ADC2, SPDS1, SPMS. Transgenic plants having over expression of polyaminessynthesis genes can gives more resistance to different abiotic stresses compare to wild type.
References
Alcazar R., Altabella T., Marco F., Bortolotti C., Reymond M., Koncz C., Carrasco P. and Tiburcio A. F. (2010). Polyamines: molecules with regulatory functions in plant abiotic stress tolerance. Planta., 231, 1237â€"1249.
Araby El M.M., El Akad S.S., Nassar A.H. and Ismail H.A. (2011). Variations in Polyamines and Growth Regulators under Different Conditions of Water Stress in Cell Suspension Cultures of Two Acacia Species. Journal of American Science, 7(12), 547-556.
Bohnert H. J. (2007). Abiotic stress. Encyclopedia of Life Science.
Cheng L., Zou Y.J., Ding S.L., Zhang J.J., Yu X.L., Cao J.S. and Lu G. (2009). Polyamine accumulation in transgenic tomato enhances the tolerance to high temperature stress. J Integr Plant Biol., 51, 489â€"499.
Edreva A. (1996). Polyamines in plants. Bulg. J. Plant physiol., 22(1â€"2), 73â€"101.
Kasukabe Y., He L.X., Nada K., Misawa S., Ihara I. and Tachibana S. (2004). Overexpression of spermidine synthase enhances tolerance to multiple environmental stresses and up-regulates the expression of various stress regulated genes in transgenic Arabidopsis thaliana. Plant Cell Physiol., 45, 712â€"722
Legocka J. and Kluk A. (2005). Effect of salt and osmotic stress on changes in polyamine content and arginine decarboxylase activity in Lupinusluteus seedlings. J Plant Physiol.,162, 662â€"668.
Liu J.H., Kitashiba H., Wang J., Ban Y. and Moriguchi T. (2007). Polyamines and their ability to provide environmental stress tolerance to plants. Plant Biotechnology, 24, 117â€"126.
Luo J., Fuell C., Parr A., Hill L., Bailey P., Elliott K., Fairhurst S.A., Martin C. and Michael A.J. (2009). A novel polyamine acyltransferase responsible for the accumulation of spermidine conjugats in arabidopsis seed. The Plant Cell, 21, 318â€"333.
Palavan U.N. (1995). Stress and polyamine metabolism. Bulg. J. Plant Physio.l, 21(2â€"3), 3â€"14.
Pang X., Zang Z., Wen x., Ban Y. and Moriguchi T. (2007). Polyamines, All-purpose players in response to Environment stresses in plants. Global science books, Plant stress.1(2), 173-188.
Walters D.R. (2003). Polyamines and plant disease. Phytochemistry, 64, 97â€"107.
Yamaguchi K., Takahashi Y., Berberich T., Imai A., Miyazaki A., Takahashi T., Michael A. andKusano T. (2006). The polyamine spermine protects against high salt stress in Arabidopsis thaliana.FEBS Letters.580, 6783â€"6788.
About Author / Additional Info: